PODS® Technology
PODS® Products
Support Literature
Introduction
What are PODS®?
- Sub-micron scale co-crystals made 100% from protein
- Contain a defined amount of a bioactive cargo protein plus a bio-inert scaffold protein
- Antioxideant and Super-Dry (with only 19% solvent content) providing unparalleled stability
- Slowly release their bioactive growth factor cargo through nanopores, generating steady-state bioavailability (near zero-order kinetics).
- Pack sizes range from 5 µg to 1mg of the bioactive cargo protein
What are PODS® used for?
- Reprogramming phagocytic cells, including monocytes and macrophages.
- Functionalizing biomaterials
- Localized sustained release in vitro and in vivo
- Generating concentration gradients
- Reducing the frequency of intervention in cell culture
- Reducing temporal concentration variability
Conventional growth factors degrade rapidly. This is wasteful (as excess growth factor needs to be added to compensate) and the dramatic swings in concentration on replenishment can stress cells. PODS® are formulated growth factors that address the limitations of conventional growth factors. PODS® are incorporated into sub-micron scale protective protein crystal lattice. This Super-Dry lattice has a remarkably low solvent content of 19% (Nature 1;446(7131):97-101) that stabilizes cargo proteins even at high temperatures.
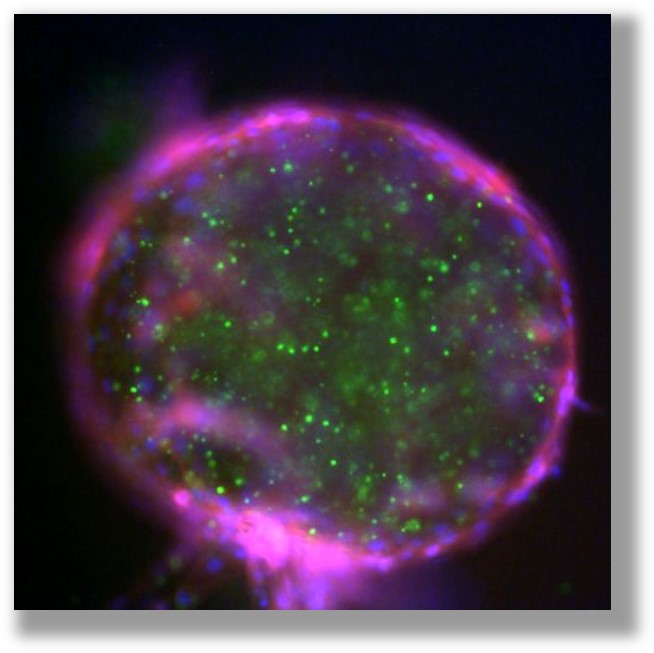
The cargo protein captured in PODS® is released by proteases (secreted by cells) which cause pores to develop in the PODS' crystalline lattice allowing the cargo growth factor to escape as a constant stram slowly. This mechanism maintains the bioavailability of the cargo protein at steady-state levels (near zero-order kinetics). The crystals can also be readily used for patterning by attachment to surfaces or suspension at specific positions in hydrogels. PODS® have a wide range of applications in-vitro (2D or 3D culture) and in-vivo.
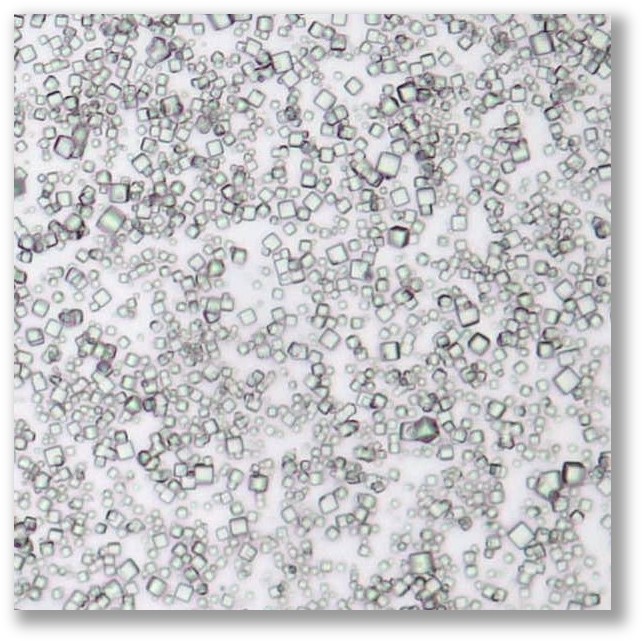

How are PODS® made?
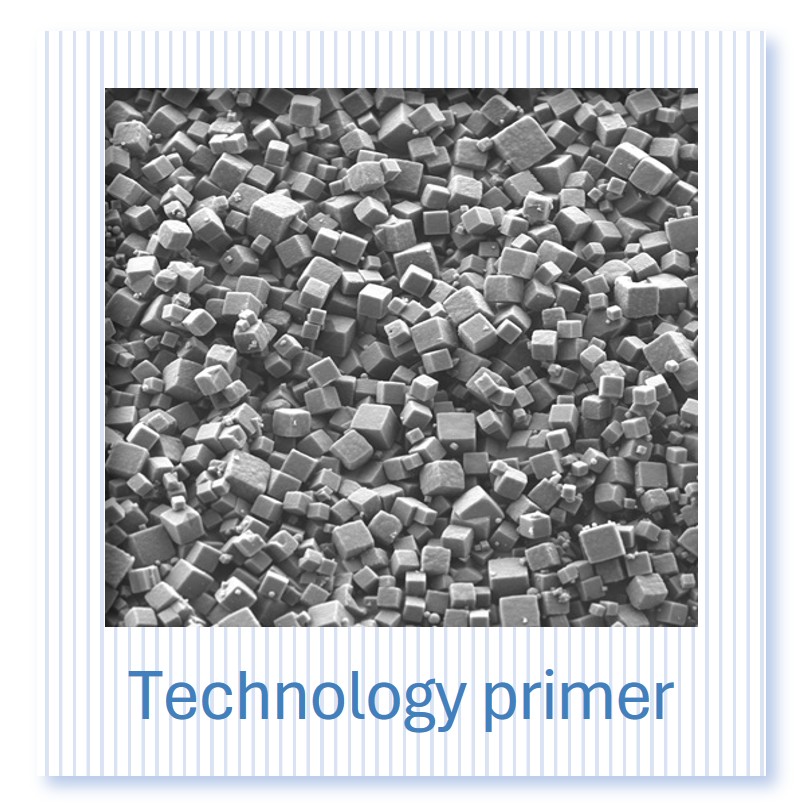
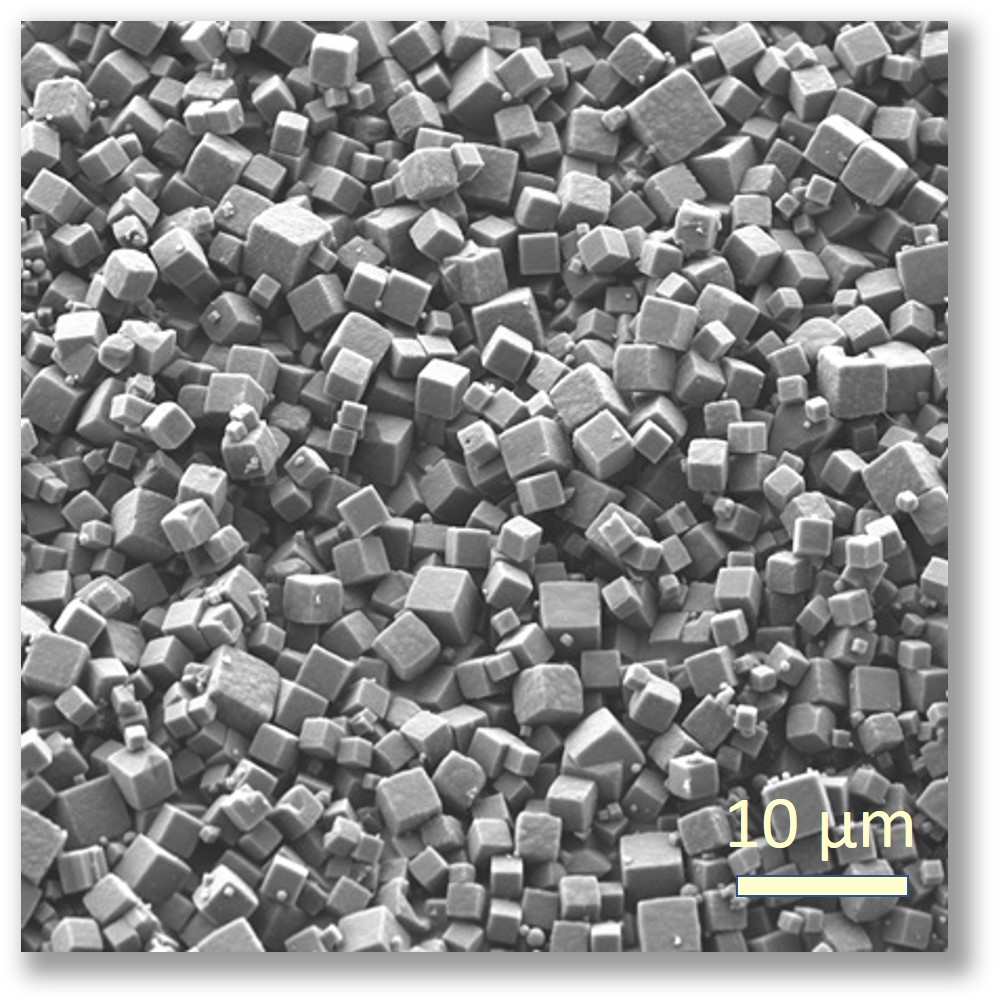
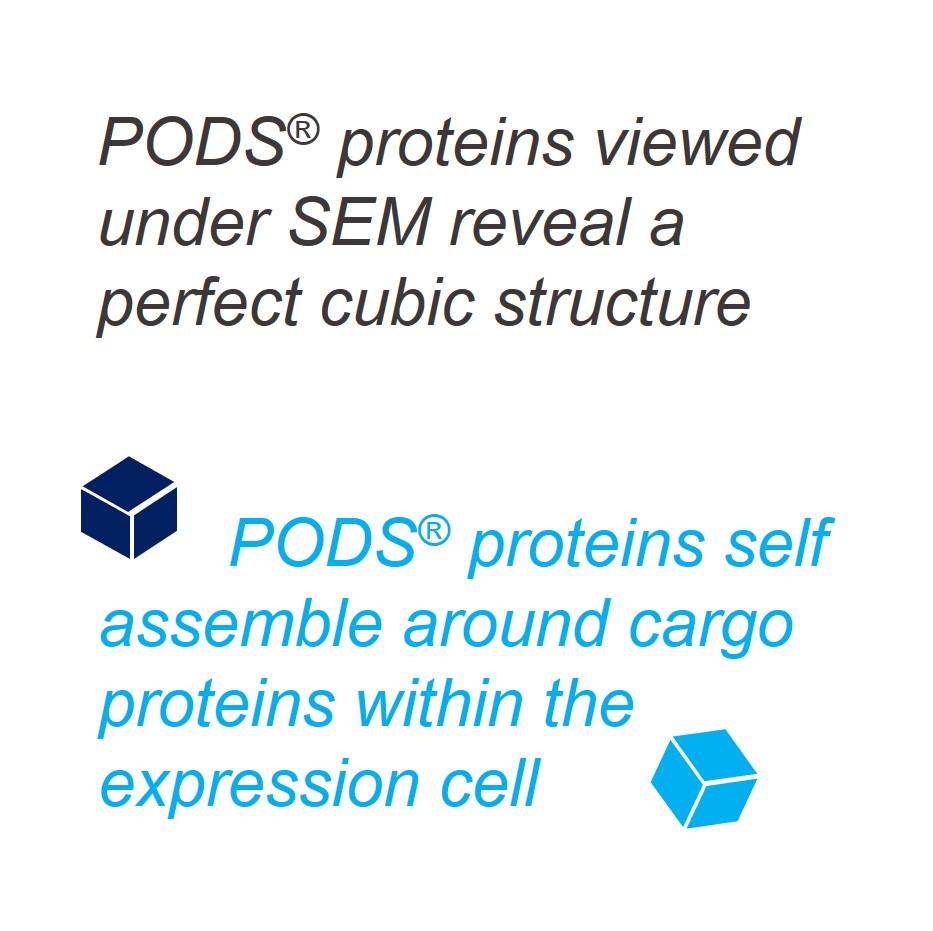
PODS® contain a cargo protein that is encased within a microcrystal formed by self-assembly of polyhedrin protein. The process of generating these crystals all occurs inside the insect expression cell and results in perfect cubic structures about 0.2 - 5 microns across. The PODS polyhedrin crystal shell also protects the active protein during purification, ensuring the cargo protein is not denatured and is therefore correctly folded and functional. Even under the light microscope, you can see the cubic structure of the polyhedrin crystal. Empty PODS crystals are transparent but refract light when loaded with cargo. For most purposes, this does not interfere with imaging.
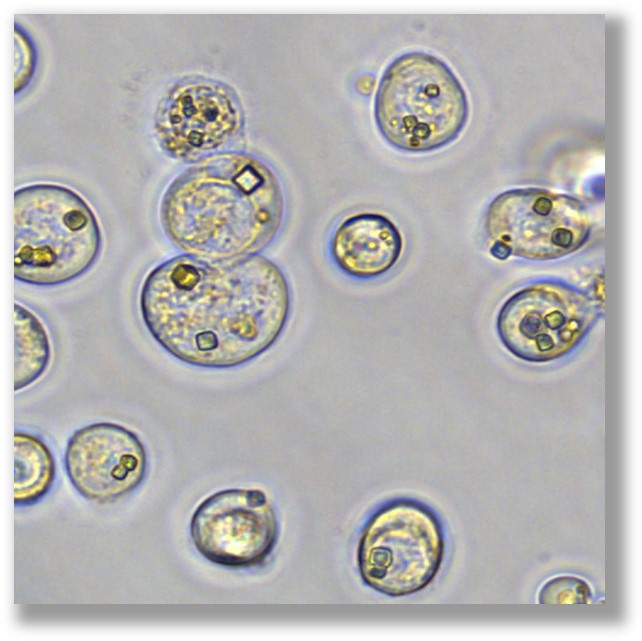

Storage Stability
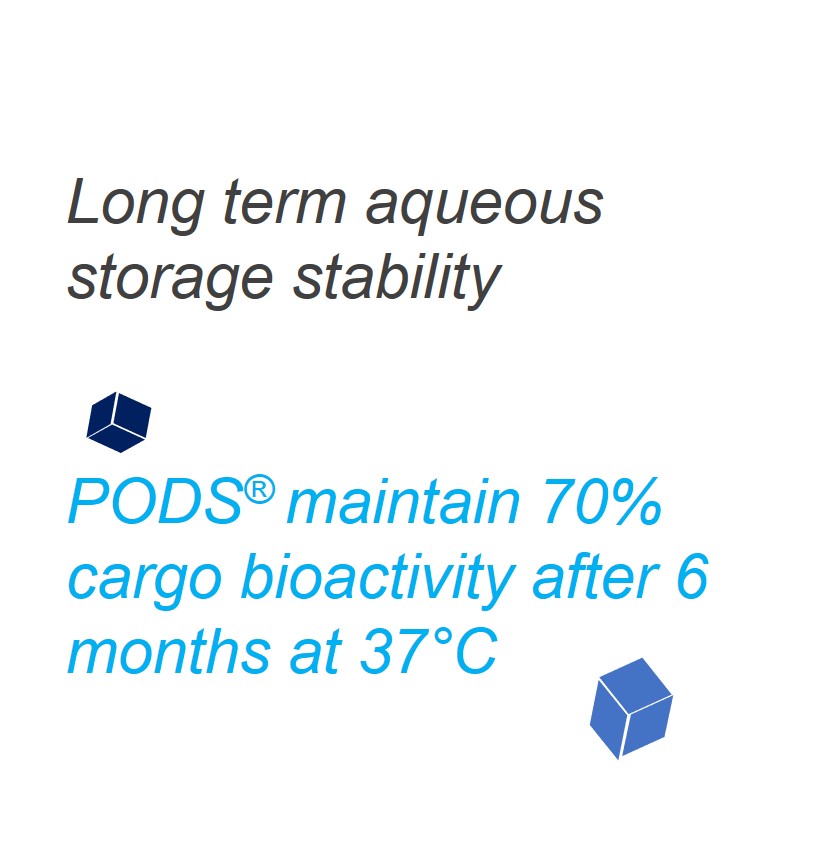
Following purification, PODS® proteins can be stored long term either dry or in liquid or hydrogel or another bio-scaffold, even at 37°C where 70% bioactivity is retained after 6 months.
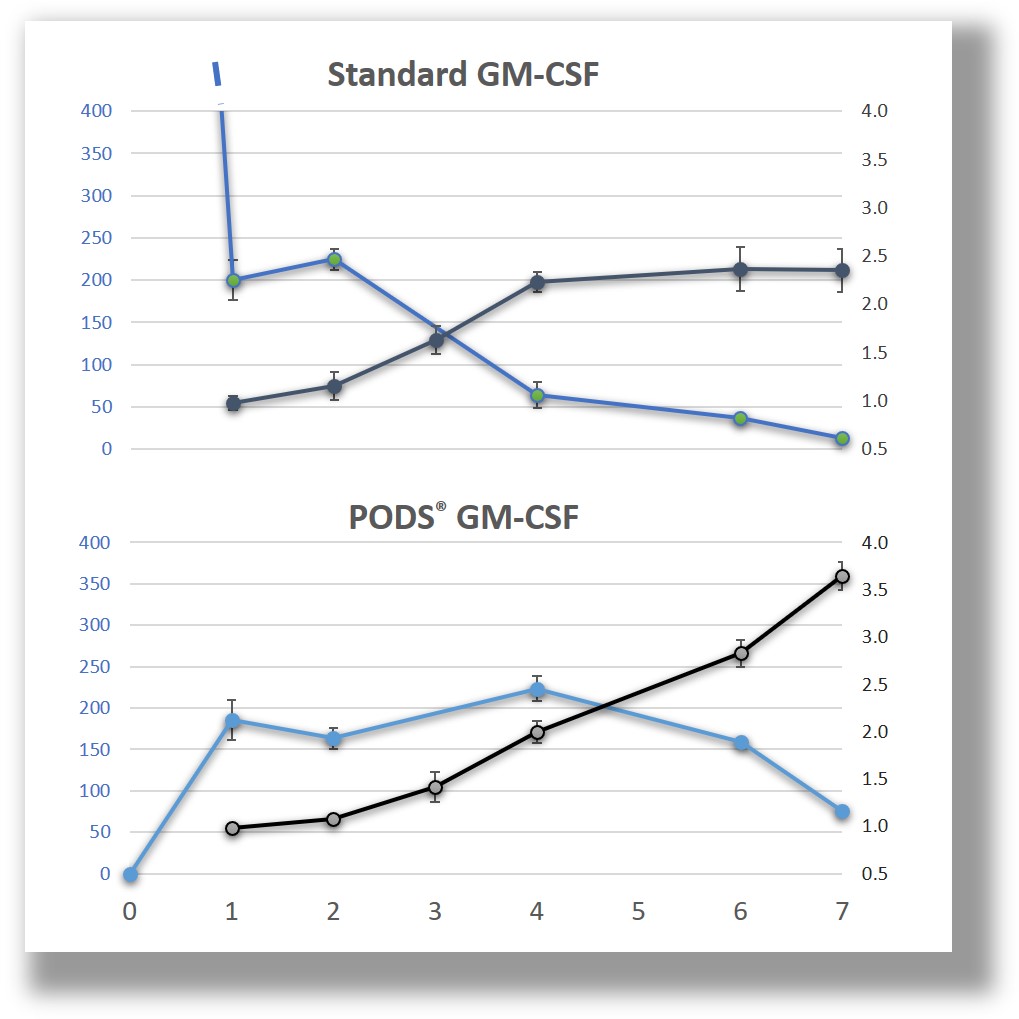
Sustained-release, steady-state bioavailability
The PODS® crystals slowly release cargo through pores formed in the polyhedrin as it is degraded by proteases released by cells. Independent, peer-reviewed analysis has shown that as a result of this sustained-release, which generates an optimal concentration zone, cells experience less stress and are consequently healthier.
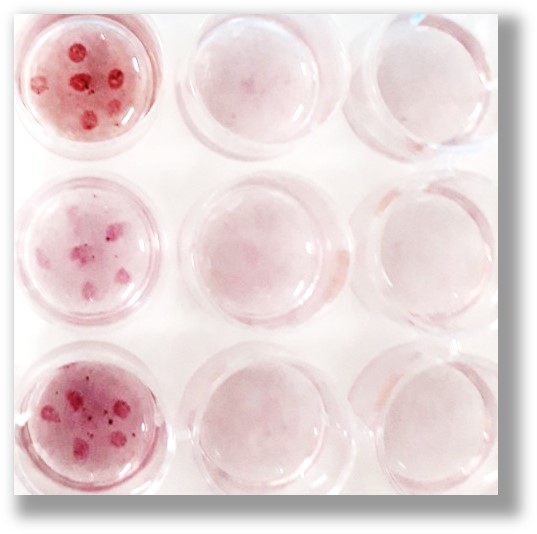
PODS® can be readily positioned to act locally
PODS® can be easily attached and immobilized to 2D surfaces and 3D scaffolds. The possibilities are limited only by our imagination. PODS can be used either naked or incorporated into bio-scaffolds and then positioned by hand or using an instrument, such as a 3D cell printer.
Attributes of PODS® growth factors:
- Extensively tested in-vitro and in-vivo
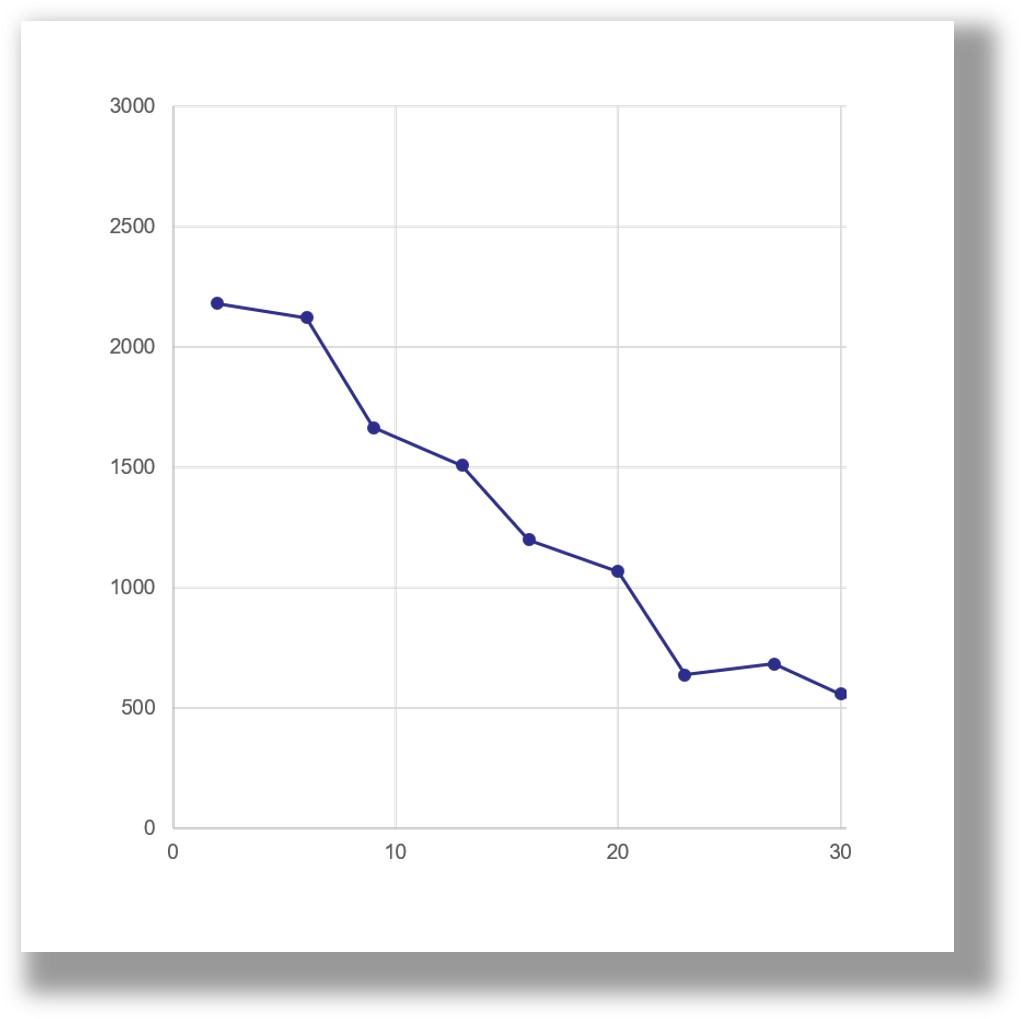
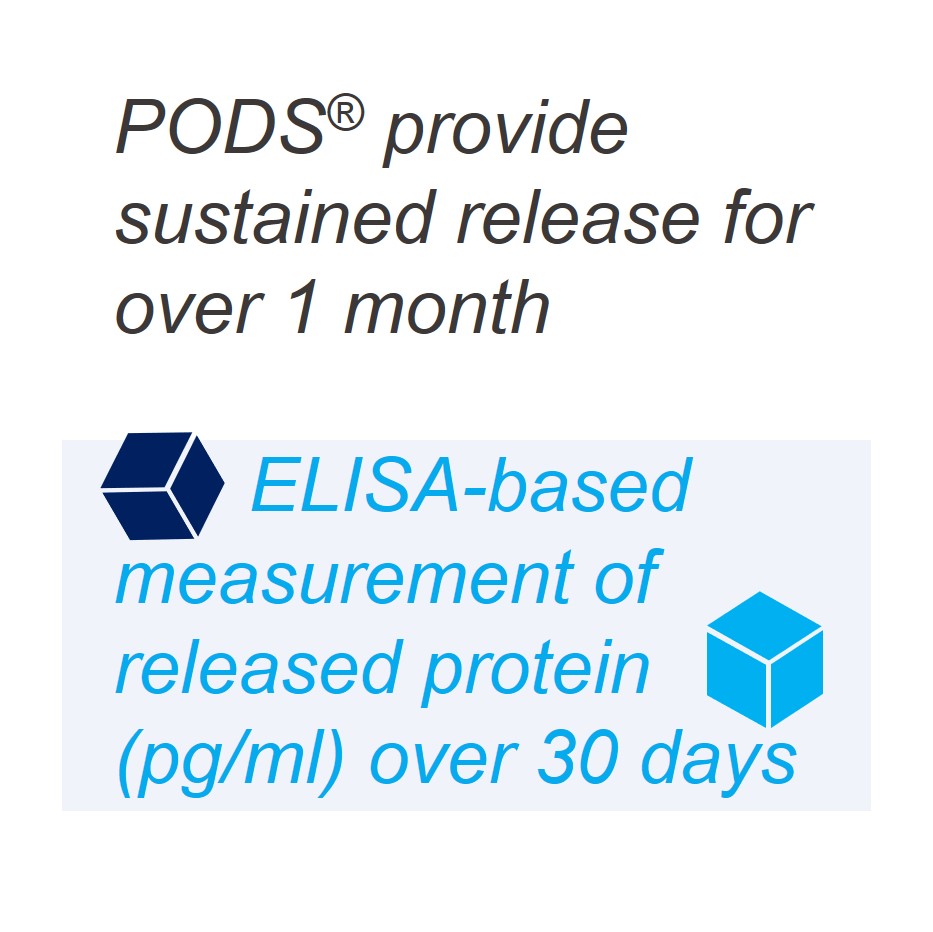
- Release of bioactive cargo demonstrated up to 8 weeks
- Withstand high-temperature manufacturing processes to create novel combination products
- Readily attaches to substrates for a desired localized effect
- Minimal batch-to-batch variation
- Produced in insect cells for important post-translational modifications
- Animal-free production
Benefits and applications of PODS® growth factors:
- Spatial and temporal control
- Reduced frequency of cell culture media changes
- Significantly stabilizes growth factor concentrations available in media for more improved cell phenotype
- Ready attachment to substrates, allowing localized deposition within a culture system
- Development of physiologically relevant concentration gradients for patterning
Comparison of conventional growth factors and PODS® growth factors
Conventional vs. PODS® growth factors |
|
Conventional |
PODS® |
|
Bioactive |
Bioactive |
| Degrades even in a sterile solution | Stable in sterile solutions |
| Heat-labile | Heat-stable over long periods |
| Difficult to localize | Readily localized - can form gradients |
| Batch-to-batch variability | Highly reproducible |
| Difficult to incorporate into biomaterials | Easily incorporates into biomaterials |
| Requires frequent replenishment | Growth factor constantly replenished from store within PODS crystals |
| $ to $$$$ - economical to very expensive | $ - economical |
Frequently Asked Questions
How big are PODS® nanoparticles?
PODS® crystals are cubic and typically 0.2-7 microns in size with a modal size of 2-3 microns.
What are PODS® made of?
PODS® are protein co-crystals. The bulk (95-99.5%) of the crystal is a polyhedrin scaffold protein. The remainder is cargo protein.
What proportion of the PODS is cargo protein
The exact amount typically varies between 0.5% - 5%. PODS® pack sizes indicate the amount of cargo that is present. For example, a 5 ug pack with a 1% cargo will contain 5 ug of cargo protein, 495 ug of scaffold protein and 500 ug protein in total.
What types of cargo molecules can you put in PODS® nanoparticles?
PODS® can only be used with protein cargos.
What is a PODS® nanoparticle's buoyant density?
PODS® co-crystals are slightly heavier than water and will settle on the surface of a culture vessel. Care should be taken when aliquoting since PODS® crystals will sink to the bottom of a tube within a few minutes. The majority of PODS® crystals will remain in suspension for up to 60 min in a 20% glucose solution (or a solution of similar density).
How stable are PODS® nanoparticles?
We have tested a variety of conditions to assess their impact on the integrity of PODS® crystals. PODS® crystals are stable at high temperatures for short periods. They are highly stable when stored in solutions between pH 6-8 and can withstand pH 3-10. Above pH 10, PODS® crystals lose their stability and dissolve rapidly (in a few hours). PODS® crystals are stable in standard cell culturing temperatures for extended periods of time (>6 months).
How is the active protein released?
PODS® protein cargo is released by proteases. Proteases may be derived from components of the solution (e.g. serum) or secreted by nearby cells. Therefore the culture system affects the amount of growth factor available in solution. In contrast to gel-encapsulated proteins (using hydrogels such as PLGA) PODS® crystals do not produce an initial burst release of cargo proteins. For naked PODS® crystals in cell culture, peak release in culture systems typically occur at day 2, then gradually diminishes. Peak-availability is not the same as peak-release-rate and depends on a number of factors including the rate of protease released by cells and the inherent stability (half-life) of the cargo protein.
How quickly is the cargo protein released from the crystals?
The rate of release, and the rate at which the cargo protein accumulates, depend on levels of proteases available, the stability of the released protein and any flushing (e.g. in vivo) or media exchange
How much should I use?
Conventional growth factors are always used in excess to compensate for their short half-lives. As a rule of thumb, you can use the same amount of PODS protein in serum-containing culture. The dynamics will be different.
Is it possible to modify the release profile?
The release of active proteins into cell culture occurs over a period of 1-3 weeks. This release period can be extended if the PODS® crystals are combined with a scaffold, which acts as a barrier to protease activity. Release rates will also be influenced by the presence of proteases in the scaffold. Bovine collagens, for example, will contain proteases.
Will PODS® nanoparticles adhere to plastics?
PODS® crystals will adhere to untreated plastics and are, therefore ideal for micro-patterning and scaffold functionalization. For sub-aliquoting, we recommend using low adherence plastics, such as low protein binding centrifuge tubes.
How often should media and PODS® nanoparticles be replaced in the culture system?
The frequency of media change depends on (1) the speed with which nutrients are exhausted or degraded and (2) the speed with which toxic metabolites accumulate. In most cell culture systems, the stability of growth factors is an over-riding issue which drives media replenishment. However, particularly in cells with high levels of metabolic activity, other factors will eventually become important once the stability issue has been addressed. We have maintained growth-factor-dependent cultures for several weeks without needing to add new PODS® crystals.
Are PODS® nanoparticles transparent?
PODS® without any cargo are isotropic crystals that do not refract light. However any cargo protein impacts on this property such that they will refract light. PODS® crystals generally do not interfere with the generation of images using imaging techniques such as phase-contrast and may also interfere with an assay that utilizes the measurement of optical density.
Can PODS® crystals have a physical impact on cell behaviour?
Physical features on a culture surface may impact the behavior of certain cells, particularly at high densities. If PODS® crystal topology is a concern, PODS® crystals may simply be incorporated into a hydrogel surface coating. Phagocytic cells (such as macrophages) will ingest PODS®. We have not observed any effect on the health of these cells. However, if this is a concern, the PODS crystals may be incorporated into a gel. Custom-made Matrigen™ Softwell plates (flat hydrogels of defined elasticity) containing PODS® crystals are available from Cell Guidance Systems.
Do PODS® cargo proteins have post-translational modifications?
PODS® crystals are made in insect cells. PODS® active proteins, therefore, contain most of the post-translational modifications that are also found in mammalian cells.
Are PODS® nanoparticles immunogenic?
The polyhedrin protein has been tested many times in-vivo and, although antibodies are generated against polyhedrin, an obvious inflammatory response has not been observed. Lack of an inflammatory response for foreign proteins is not without precedent: silk fibroin protein from the silkworm B. mori is commonly used for surgical stitching and Botulinum toxin (Botox) is widely injected in cosmetic procedures.
Are PODS® proteins equivalent to standard growth factors?
The stability of standard recombinant growth factors varies significantly. The most labile, such as FGF-1, have a sera half-life measured in minutes limiting their utility. The stability of PODS® growth factors that are encased in PODS® crystals is much longer but once a molecule is released will have the same half-life as their standard recombinant counterparts. This may be advantageous for 3D applications as it reduces the path length
The amount of available growth factor in a culture system is a function of the speed of release from the crystal and the subsequent stability. In the first few hours of a culture containing only newly added PODS® crystals, there is little growth factor protein available for the cells. Significant amounts of growth factor protein are available after one day. The initial lag of protein availability may be corrected if necessary by adding a small amount of standard recombinant growth factor.