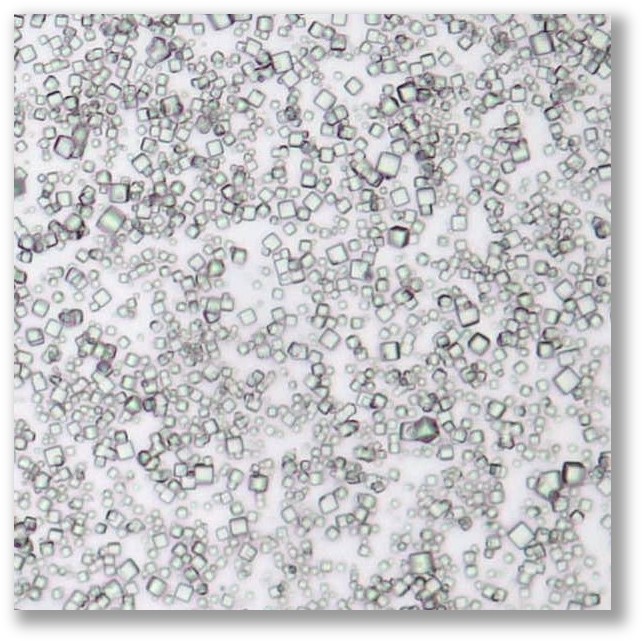
Overview
POlyhedrin Delivery System (PODS®) are protease-responsive matrix microparticles that provide the sustained release of therapeutic proteins such as cytokines.
PODS® achieves sustained release by utilizing a specific polyhedrin protein crystal to encase and protect a chosen cargo protein.
Cancer
Study Partners: Dr Nirk Quispe Calla and Dr Hong Zeng, Stanford University, California
Cancer drugs tend to have high levels of toxicity which could be reduced by better targeting to the diseased cells. The multi-faceted behaviour of monocytes and their macrophage progeny suggest their utility to courier particulate drugs to the tumour microenvironment. PODS® crystals are efficiently taken up by monocytes and subsequently released in a bioactive form by macrophages. Most recently, we have shown that intravenously injected PODS® crystals deliver therapeutic doses to tumours whilst retaining remarkably low serum levels.
The hypothesis of PODS MP-mediated drug delivery. Drugs delivered to cancer from the blood need to be able to traverse the endothelial barrier of the blood vessel wall and permeate the tumour environment (TME). The TME is particularly challenging for fibrotic tumours and for larger drugs, such as proteins, which diffuse more slowly. Even TME targeting technologies such as antibody-drug conjugates (ADCs), rely on passive delivery across the endothelium and ECM. (A) The delivery problem is exacerbated by the fact that many protein drugs are rapidly cleared from the serum by the filtering effects of the kidney (e.g. IL-2 has a half-life of 5 minutes in serum). This can necessitate high drug doses which increase the risk of toxic off-target side effects. (B) Using PODS addresses these issues. Any drugs released in the serum from IV-injected PODS are cleared and unable to accumulate. PODS are ingested by monocular phagocytic cells (MPs) such as momocytes which actively infiltrate the TME. Here, the slowly released drug is outside the filtering effect of the kidney and accumulates
Osteoarthritis
Study Partners: Prof Andrew McCaskie, Dr Frances Henson, University of Cambridge, UK.
Osteoarthritis is a major clinical and economic burden. In the UK, for example, 29% of adults over 45 have OA, of which 9% is severe (Arthritis Research UK). This is caused by damage to the cartilage tissue that protects the ends of bones in joints. Once this tissue is damaged, the joint becomes painful. Eventually, the joint may need to be replaced with a prosthetic. Surprisingly, there are no approved therapies which can slow down the progression of osteoarthritis. Our work with Cambridge University is developing a new method to treat the cartilage surface defects that lead to osteoarthritis, using growth factors delivered via PODS® crystals. In this study, we have tested specific PODS® growth factors, both in vitro and in-vivo for their potential to promote cartilage surface defect repair.
Bone Regeneration
Study partners: Prof Hajime Mori, Kyoto Institute of Technology, Japan
Musculoskeletal injuries and diseases are highly prevalent, accounting for around a fifth of all medical visits and impose a significant societal and economic cost on healthcare providers. Surgical orthopaedic intervention is increasingly common and frequently relies on the use of implant devices and grafts to restore musculoskeletal functionality. We are conducting pre-clinical studies to determine the utility of PODS® crystals for a specific medical indication requiring bone regeneration.
Age-Related Macular Degeneration
Study partners: Dr Petr Baranov and Dr Julia Oswald, Schepens Eye Institute
Loss of cells lining the inside of the eye due to disease leads to loss of central vision. Replacing these cells with iPSC-derived cells has the potential to reverse the effects of AMD. Researchers at Schepens Eye Institite at Harvard University have been using PODS® containing BDNF and GDNF to improve the quality of iPSC-derived retinal organoids. They found that using PODS® produced higher quality organoids - possibly because this requires fewer interventions. Petr and Julia are dissociating the cells prior to injecting into eyes along with PODS® proteins to promote implanted cell survival and engraftment.
Cochlear Implant Integration
Study partners: Dr Aki Matsuoka, Northwestern University
Cochlear Implants are now widely used to restore hearing loss. The performance of the implant depends, in part, on the level of integration of the implant with neuronal cells lining the inner ear which conduct nerve signals to auditory regions of the brain. Dr Matsuoka is exploring the use of PODS® containing neurotrophic growth factors to coat the cochlear implant. It is anticipated that these will establish a gradient that will attract neuronal cells resulting in improved cochlear implant integration.
Infection
Study Partners: Prof Ian Goodfellow, University of Cambridge, UK
Cytokines are able to modulate the immune response to infection. We are examining the utility of PODS® crystals to reduce infectivity rates for infectious diseases such as norovirus to provide a prophylactic and therapeutic drug to control disease outbreaks.
Learn more at www.cuboid.bio