Local, longer: Sustained drug release
To reduce the required frequency of drug administration, technologies have been developed to offer prolonged therapeutic effects, minimizing systemic side effects. These technologies are particularly useful for chronic conditions, localized diseases, and situations where continuous protein delivery is beneficial. Technologies that have been developed for sustained, localized release include:
Biodegradable Polymeric Implants
These are devices made from polymers that gradually degrade in the body, releasing encapsulated protein drugs over a prolonged period. The rate of degradation—and thus drug release—can be controlled by the choice of polymer and its properties. Commonly used biodegradable polymers include polylactic acid (PLA), polyglycolic acid (PGA), and their copolymer, polylactic-co-glycolic acid (PLGA).
Hydrogels
Hydrogels are three-dimensional, hydrophilic polymer networks capable of absorbing large amounts of water or biological fluids. They can be engineered to release encapsulated proteins in a controlled manner, either through degradation of the hydrogel matrix or diffusion of the protein through the hydrogel. Stimuli-responsive hydrogels can further fine-tune the release profile in response to environmental cues such as temperature, pH, or enzymes (e.g. proteases). PeptiGel® from Cell Guidance Systems has been validated in animal models for sustained-release applications.
Microspheres, Nanospheres and microgels
These spherical particles are typically made from polymeric materials and hydrogels. They can encapsulate proteins and release them slowly as the particle-matrix degrades or as the protein diffuses out. The size, composition, and surface properties of the spheres can be tailored to control the release rate and target the delivery to specific tissues.
Liposomes
Liposomes are vesicles formed from phospholipid bilayers that can encapsulate protein drugs. They can be designed to release their payload slowly as the liposomal membrane undergoes biodegradation or fusion with cell membranes. Surface modification of liposomes with polymers like polyethylene glycol (PEG) PEG can further prolong their circulation time and modify release kinetics.
In Situ Forming Implants (ISFIs)
ISFIs are injectable formulations that solidify or gel upon administration into the body, forming an implant that releases encapsulated proteins over time. The transition from liquid to solid can be triggered by changes in temperature, pH, or exposure to water or body fluids. ISFIs offer the advantage of a minimally invasive administration compared to pre-formed implants.
Electrospun Fibers
Electrospinning is a technique used to create ultrafine fibres from polymer solutions or melts. Proteins can be encapsulated within these fibres, which can then be fabricated into mats or scaffolds. The release of proteins from electrospun fibers can be controlled by the composition of the fibres, their structure, and the degree of cross-linking within the polymer matrix. This technology allows for the creation of scaffolds with specific mechanical properties and degradation rates, making it suitable for tissue engineering applications where sustained protein delivery is desired alongside tissue support and regeneration.
Osmotic Pumps
Osmotic pumps are small, implantable devices that use osmotic pressure to deliver drugs at a constant rate. They can be filled with a solution or suspension of the protein drug, which is then released through a small, semipermeable membrane. The rate of drug delivery can be controlled by the osmotic gradient and the properties of the membrane. Osmotic pumps are particularly useful for delivering small, consistent doses of protein drugs over extended periods.
Microneedle Patches
Microneedle patches consist of arrays of tiny needles that can penetrate the skin’s outer layer to deliver drugs directly into the dermal tissue. Proteins can be coated on the needles or encapsulated within biodegradable microneedles that dissolve upon insertion. This method provides a minimally invasive way to achieve localized, sustained release of protein drugs with the potential for self-administration.
Biodegradable Nanoparticles and microparticles
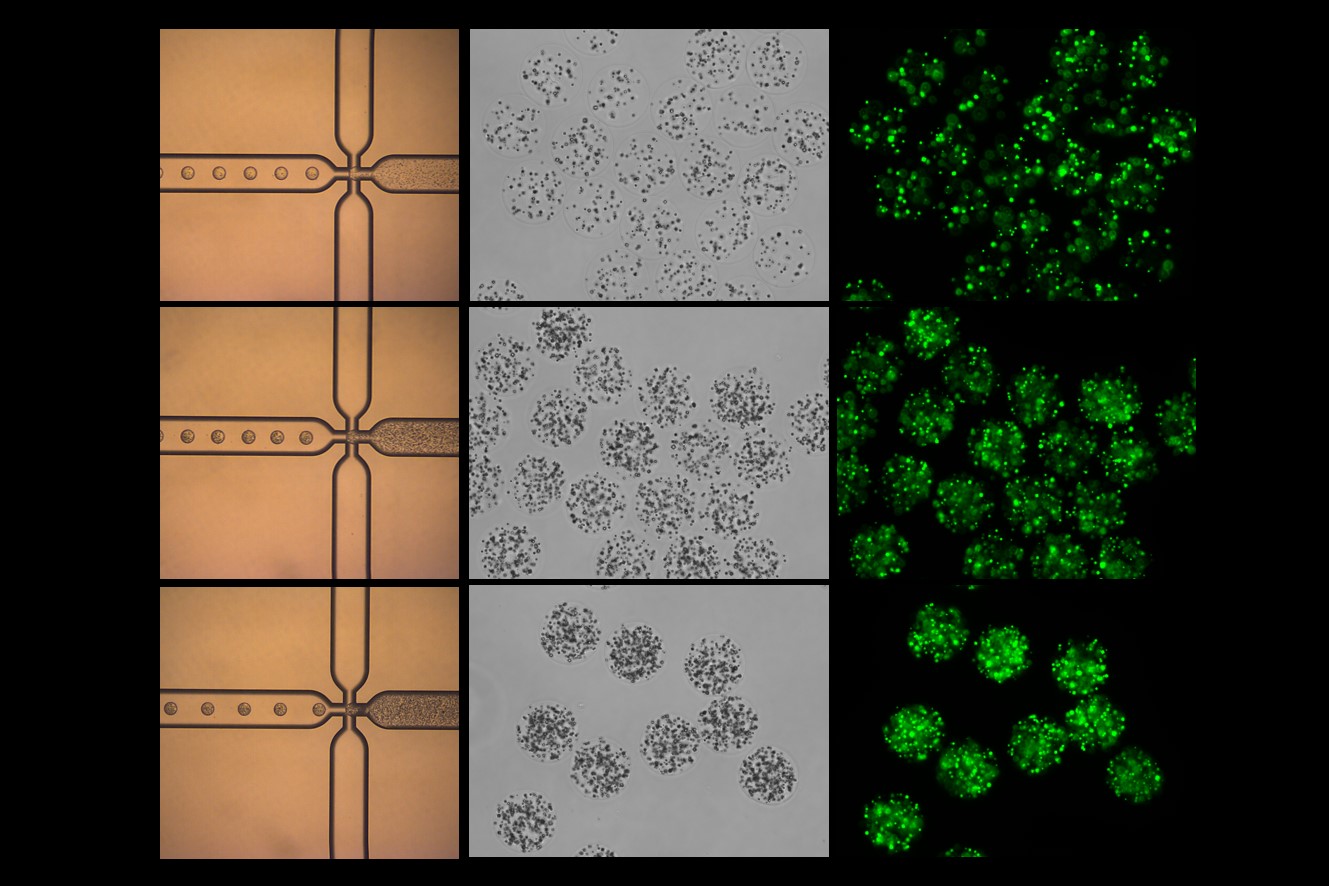
Similar to microspheres, nanoparticles made from biodegradable materials can encapsulate protein drugs and release them as the particles degrade. The small size of nanoparticles allows for enhanced tissue penetration and the possibility of targeting specific cell types by modifying the surface of the particles with targeting ligands. PODS® microparticles from Cell Guidance Systems use a crystalline protein scaffold which degrades over time under the action of proteases to sustainably release cargo.
Cell-based Delivery Systems
Genetically engineered cells can be used as living factories to produce and release therapeutic proteins directly at the site of implantation. These cells can be encapsulated within biocompatible and biodegradable matrices that protect them from the immune system while allowing the diffusion of proteins into the surrounding tissue. This approach can provide a continuous source of proteins and is being explored for applications such as insulin production in diabetes and factor VIII production in haemophilia. Phagocytic cells can also be loaded up with protein drug containing microparticles, such as PODS, to provide sustainable release.
Enzyme-responsive Systems
By exploiting the overexpression of certain enzymes in specific diseases or tissues, it’s possible to design drug delivery systems that release their protein payload only in the presence of these enzymes. This approach ensures that the drug is released only at the site of disease, minimizing systemic exposure and side effects.
Light-responsive Systems
These systems use light-sensitive materials to control the release of encapsulated proteins. By exposing the delivery system to light of a specific wavelength, it’s possible to trigger a change in the material (such as degradation or a change in permeability) that results in the release of the protein drug. This method allows for precise spatial and temporal control over drug release and can be particularly useful for applications where external control over the therapy is desired.
Bioresponsive Hydrogels
These are advanced hydrogels that can respond to specific biological signals, such as changes in glucose levels, pH, or the presence of specific biomolecules. By designing hydrogels that respond to signals relevant to the disease state or target tissue, it’s possible to achieve highly targeted and controlled release of protein drugs. For example, in the case of diabetes, hydrogels that respond to elevated glucose levels can be used to release insulin in response to the patient’s fluctuating glucose levels, mimicking the natural response of the body. This approach can significantly improve the efficacy and safety of treatments by ensuring that drugs are released only when needed and in the appropriate amounts.
IMAGE: Alginate microspheres loaded with GFP-containing PODS protein crystals Credit: Cell Guidance Systems
Learn more about powerful technologies that are enabling research:



