Controlled protein drug delivery
When proteins are used therapeutically, each protein and its target pair present unique challenges for delivery. Placing proteins in the right place (targeting) for a sufficient period (sustained delivery) to achieve efficacy requires solutions appropriate to each drug.
Fundamentally, there are only three factors that need to be considered for a drug: persistence, potency, and specificity. All other considerations flow from these basic criteria. A drug’s half-life depends on inherent stability, metabolic rate and any filtering (e.g. from serum by the kidneys). The potency of a drug is a measure of how much and for how long it is needed to achieve an effect. A drug’s specificity reflects how restricted its effects are to the target tissues, cells and pathways
Small proteins, such as cytokines, generally have short half-lives and can present more of a challenge than parge proteins, such as antibodies, for delivery. The route of administration can make a big difference to availability. For example, high dose IL-2 administered intravenously has a half-life of around 5 minutes primarily due to the filtering effects of the kidneys. However, if the same drug is administered intra-peritoneally, this creates a reservoir away from the filtering effects of the kidneys producing a significantly increased half-life.
Proteins with the highest levels of persistence, potency and specificity are the easiest to administer. The earliest protein drugs such as insulin achieved therapeutic efficacy without modification of the active pharmaceutical ingredient (API) as the basic protein scored well in these three areas. Insulin has since benefited from innovations in delivery such as sustained release formulations (e.g. Lantus) and hybrid closed-loop glucose monitoring and release systems that provide drug delivery that people can more easily live with.
Whilst insulin was feasible without modification, for many other proteins, release systems are a prerequisite for efficacy. Innovation around sustained release systems has developed a range of options for other drugs. These include:
Mechanical pumps
In addition to the delivery of insulin, portable mechanical pumps, or onboard delivery systems (OBDS) have been developed to deliver protein drugs. These include Neulasta (PEGylated G-CSF) for stimulation of granulocyte production and evolocumab an antibody that binds to PCSK9, where the antibody's therapeutic effect is to lower LDL-cholesterol and reduce risk for atherosclerosis.
Osmotic pumps
These are passive drug delivery systems that rely on osmotic forces to expel the drug from a capsule. Osmotic pumps' simplicity means they are relatively small and robust and can therefore be implanted. Alzet manufactures osmotic pumps for use in animals.
Long-acting injectables
Proteins can be stabilized by, for example, conjugating another chemical such as the addition of PEG (PEGylation) to G-CSF to create the drug Neulasta. Individual amino acids can be altered to increase stability (e.g. Lantus, Longr3-IGF) and proteins can be fused to a more stable larger protein such as IgG1 (Enbrel).
Incorporating the API into a carrier system can significantly alter the availability and targeting of the drug. A huge range of controlled drug delivery systems have been developed for delivering proteins. These typically use nanoscale materials to alter the stability of a drug, or slowly release the drug and can provide passive or active targeting. These new systems can provide better-controlled drug release than traditional excipients to better maintain drug concentrations within the therapeutic window.
Amongst the most long-lasting formulations, dissolution-controlled drug delivery systems can either be reservoir or matrix based. Reservoir systems encapsulate drugs in nanoscale polymeric coatings. As each capsule’s wall is breached it rapidly releases its cargo drug, but since there are many capsules which will degrade at different rates, this creates a sustained release effect. Matrix-based drug delivery systems typically use a nanoscale polymer matrix in which the drugs are embedded. As the matrix degrades, the cargo is released such that each nanoparticle slowly releases its cargo.
Diffusion-based systems include drugs embedded in hydrogels. The hydrogel remains intact but the cargo protein slowly diffuses out. The rate of diffusion can be calculated based on Fick’s laws of diffusion.
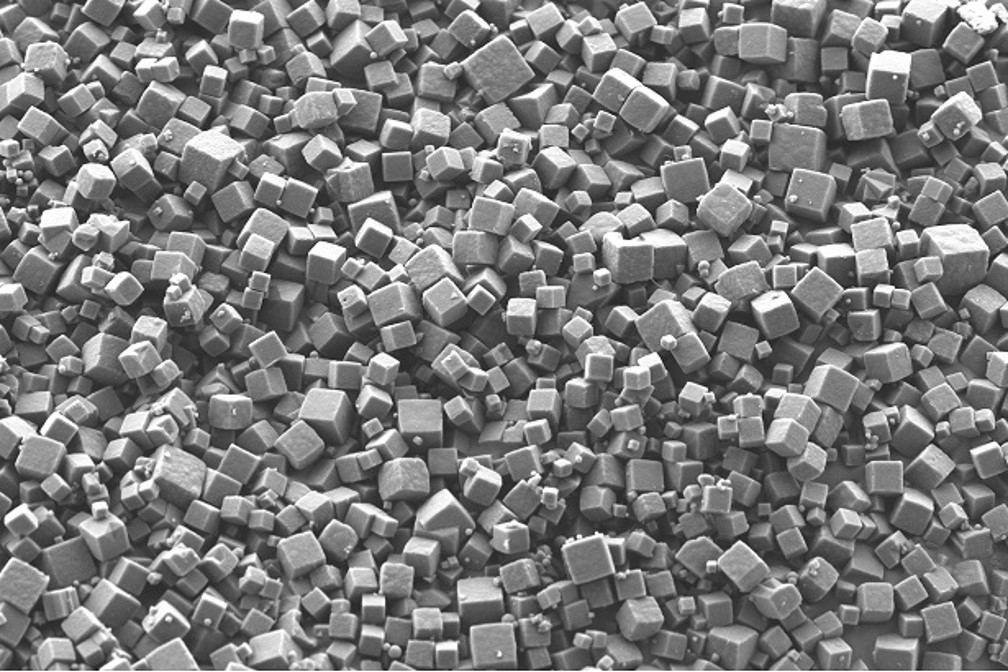
The PODS® drug delivery technology places cargo proteins within a protein crystal matrix (pictured above). This unique technology combines the characteristics of several other drug technologies. It is a responsive, dissolution matrix-based system in which degradation of the protein matrix of the PODS® crystal by resident proteases leads to the release of the cargo protein. However, this matrix degradation is not uniform and, similar to the way glaciers can develop internal flows of water, cargo molecules “frozen” in the matrix are steadily released to diffuse out through deep channels created in the material.
Innovation in drug delivery is just as important as innovation in the API. Drugs that are now poorly tolerated can be transformed into much safer treatments. Many drugs with poor drug profiles are being improved and many others are reaching the clinic due to innovations in delivery.
IMAGE PODS crystals



