Primary Human Hepatocytes - Plateable Grade
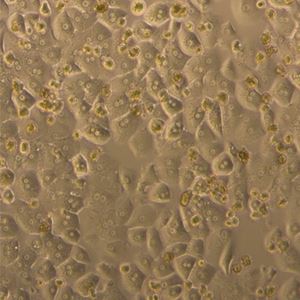
Product Description
Plateable Human Hepatocytes. Highest quality. Comprehensive QC. Samples available
Note: Samples are available for evaluation with the first order. The minimum order for this product is 10 vials. Please ask for a quote for >100 vials.
Primary human liver cells of the highest grade. Primary human hepatocytes are the only faithful liver model which is validated on Transporter Expression, CYP activity, Drug response and HBV uptake. These cells enable in vivo correlations from in vitro data to form conclusions regarding a candidate drug’s effect on the liver.
Suitable for a variety of assays including:
-
Assessing drug-drug Interaction assays
-
Metabolism studies
-
Drug influx and efflux studies
-
Compound uptake
-
Hepatotoxicity assays
-
Hepatic clearance
-
HBV/HCV replication assays
| Product Details | |
|---|---|
| Cells per vial |
> 5 million |
| Pathogen testing |
Tested negative for HBV, HCV and HIV. No bacterial or fungal overgrowth |
| Viability |
>75% |
| Monolayer formation |
Formation of a confluent monolayer at 350,000 cells/well (24 well plates) within 24 hours. |
| Monolayer survival |
Up to 5 days |
| BCA protein content |
200 ug/cm2 on a monolayer |
| Additives |
Thawing medium, percolation medium |
| Metabolic parameters tested |
Qualification is based on literature-based average and standard population deviation for: CYP2D6, CYP2C9, CYP2B7, CYP3A4, CYP3A (general) UGT2B7, UGT1A9, UGT1A4, UGT1A6 Phase II (combined, general non-specific test) |