Primary human hepatocytes functionally tested to perform in key hepatotoxicology assays. Primary human hepatocytes remain the gold standard for in-vitro assays to predict the in-vivo effect of compounds and candidate drugs.
Primary human hepatocytes tailored for in vitro toxicology assays
Grading
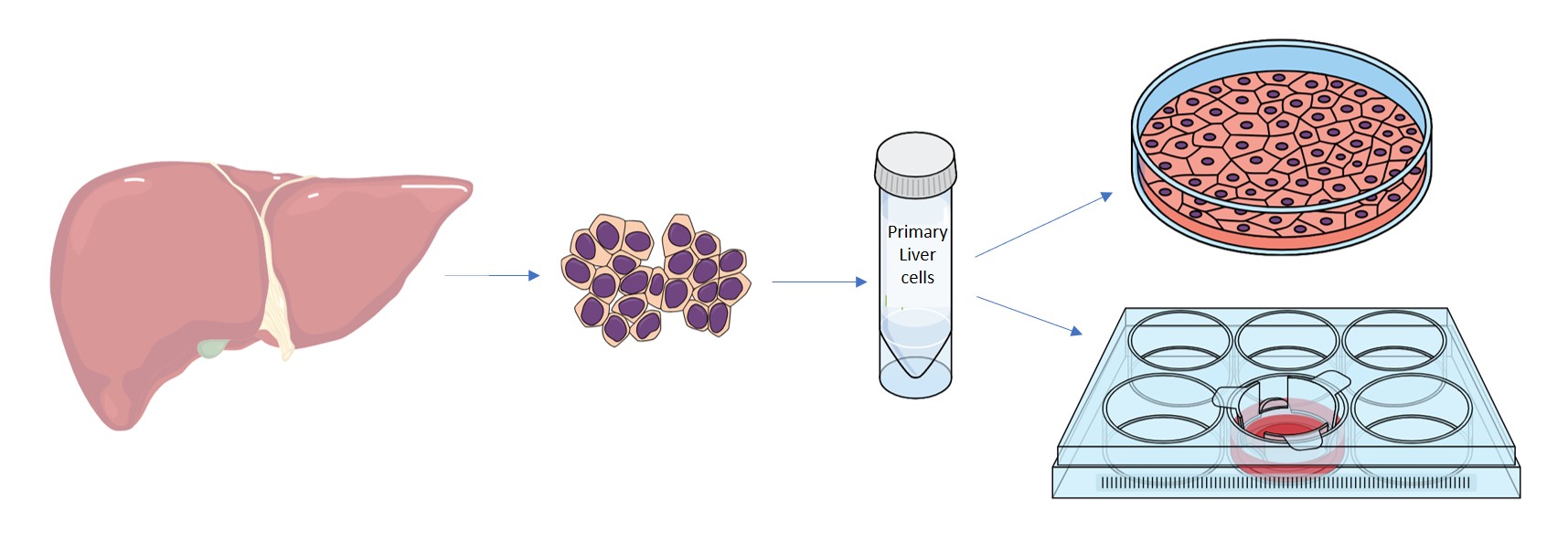
During the preparation of primary human hepatocytes, process intensity, yield and cell quality are related. As cell yield rises during the manufacture of primary liver cells due to increased process intensity, hepatocytes progressively lose key attributes, such as the ability to form a monolayer. With the varying grades, the utility of the cells for different assays (see table below) also changes. We offer hepatocyte cells in three grades to maximize their value, dependent on your specific assay requirements.
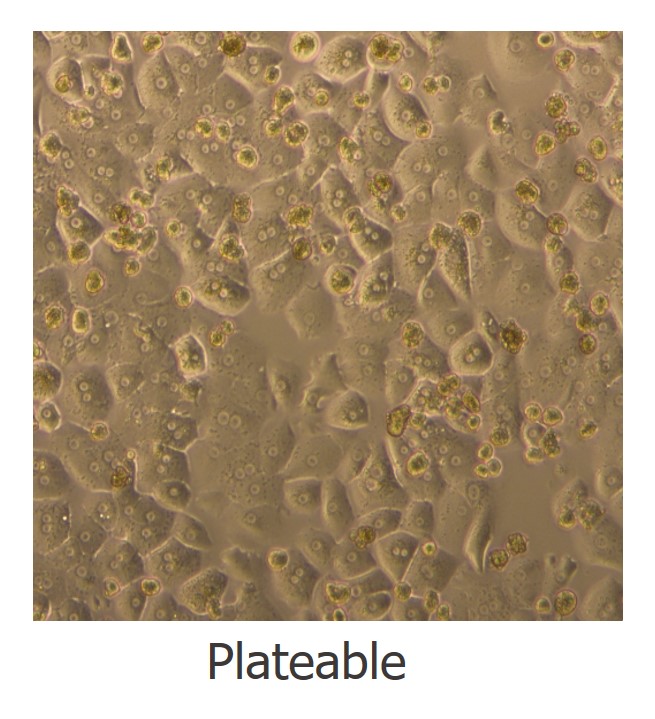

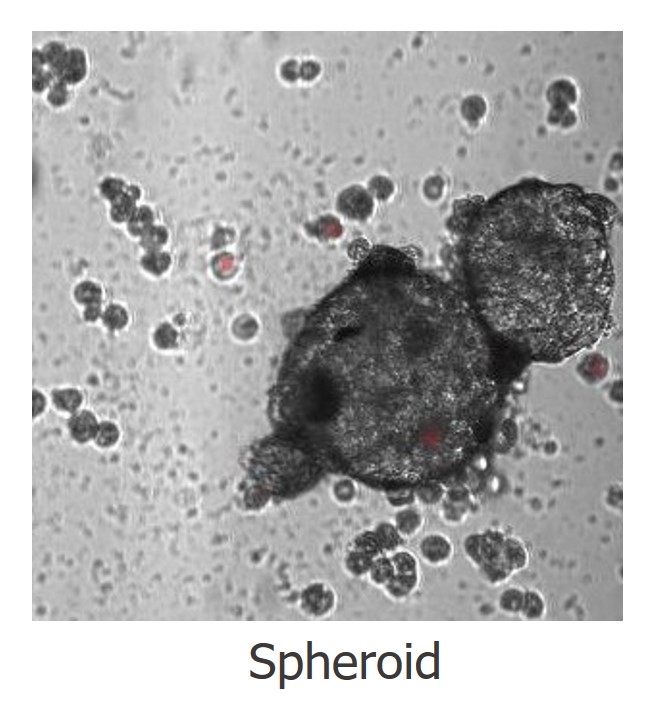
Primary Human Hepatocytes: Grade and Utility
|
|
|
HH1 - Plateable
|
HH2 - Suspension
|
HH3 - Spheroid
|
|
Overall quailty
|
Highest |
Intermediate |
Spheroid grade |
|
Viability
|
>80% |
>80% |
>80% |
|
Cells per vial
|
> 5 million |
> 5 million |
> 1 million |
| Culture Method |
Monolayer |
Suspension |
Spheroid
|
|
Metabolic characterization
|
Comprehensive |
Comprehensive |
None |
| Assay range |
Diverse - see product page |
Hepatic clearance assays,
short-term metabolic studies
|
Hepatotoxicity |
| Donor Pooling |
None |
>10 pooled |
None
|
Pooled cells are produced under a license.