Exo-spin™ midi
Product description
Exo-spin™ midi uses a simple 5-step protocol for the purification of exosomes provides reproducible results. See User Guide for details
Exo-spin™ products provide a proven, rapid and reliable way of generating high-quality purified exosomes suitable for a range of research applications.
Exo-spin™ technology is based on size exclusion chromatography (SEC), which is reliable for exosome isolation. A concentration step (using Exo-spin buffer or concentration columns) is recommended if a starting sample has low exosome-content, such as cell culture media, urine, saliva etc. Without this important step, a lower exosome recovery will be obtained. Exo-spin™ provides a simple protocol which allows you to purify your sample in less than 2 hours with consistent and reliable results.
The EX04 Exo-spin™ product contains packed and equilibrated ready-to-use SEC columns.
The EX04 Exo-spin™ kit has been developed to process up to 500 ml of cell culture medium, saliva, and urine (when used in conjunction with a concentration step, e.g. precipitation). 1 ml of blood samples (plasma and sera) may be added onto the column directly.
Do I need to order buffer as well as columns?
The Exo-spin™ midi product provides flexibility in processing between 0.5 ml of blood and 500 ml of cell culture media. For blood, which contains high concentrations of exosomes and proteins, the samples can be added directly to the columns (cat no EX04). For culture media, saliva, urine and other samples that contain lower concentrations of exosomes, the sample should be concentrated by precipitating with the buffer (cat no EX06) before applying to the columns.
The buffer is used at a ratio of 1:2 with the culture media. For example, if you have a 50ml sample, you will need 25 mil of buffer. If you have any questions, please use the chat function on the bottom right of this page.
Iterative loading?
The columns can be used, flushed and reloaded with aliquots of the same sample. This enables larger sample volumes to be purified on a single column.
Exo-spin™ midi column - How much sample can I load? |
||||
|
Are you using a precipitant/concentrator device |
no |
yes |
no |
yes |
|
Are you using Iterative loading? |
no |
no | yes |
yes |
|
sera and plasma |
1 ml |
5 ml |
5 ml |
25 ml |
|
culture media, urine, saliva |
1 ml |
500 ml |
5 ml |
2500 ml |
Table 1. The maximum sample size that can be loaded onto a column will depend on the sample type and whether the sample is concentrated by precipitation or loaded iteratively.
Exo-spin™ column and resin pore size
The column bed volume in EX04 Exo-spin™ midi-columns is 10 ml, allowing for 1 ml of volume to be loaded onto each column.
The pore size within the resin is approximately 30 nm to attain a highly pure exosome elution. All other molecules (e.g. proteins, lipids) which are smaller than 30 nm will enter into the pores and remain trapped in the column.
Highest recovery and purity
Exosomes in a range 30-200 nm will elute in the first fraction. 99.5% other proteins and lipids will be retained in the column and will elute later than the exosomes, ensuring a highly pure sample ready for downstream application.
Reproducibility
All our columns are manufactured in our laboratory to ensure a high reproducibility between each lot. As proof of reproducibility and batch-to-batch consistency, a large number of peer-reviewed scientific papers have been published describing the use of Exo-spin™.
Storage
Upon receipt, store purification columns and Exo-spin™ Buffer at 4°C. All other components should be stored at room temperature (15°C - 25°C).
Frequently Asked Questions (FAQs)
For any additional questions, please refer to FAQs document below.
If you are just starting to work with exosomes, consider our starter pack and get in touch with us for advice through the chat function on the bottom right
We designed the ideal starter pack to guide your exosome research. The starter pack includes the exosome purification kit of your choice, exosome validated antibodies, and NTA profiling analysis. Complete details can be found in the product page here.
See other Exo-spin™ columns
Product data
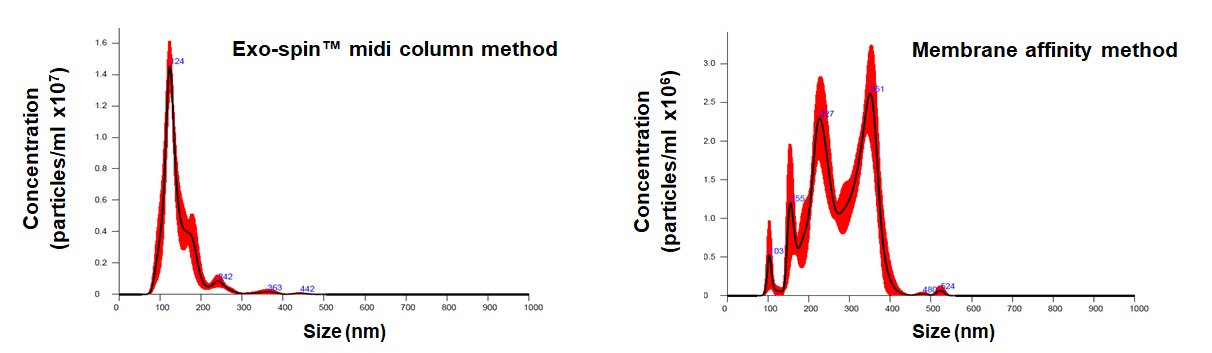
EX04 Exo-spin™ midi columns comparative data
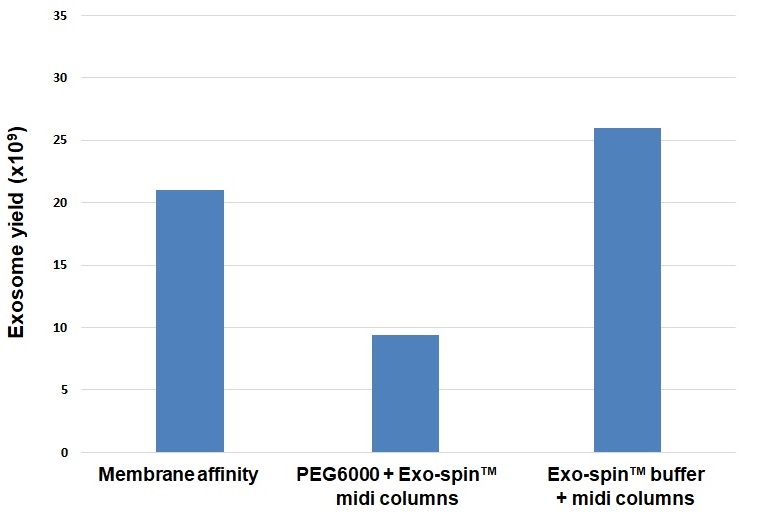
Comparison of total exosome recovery from 10 ml conditioned cell culture medium. The total particle yield obtained with Exo-spin™ buffer + Exo-spin™ midi columns is greater than the yield obtained using the membrane affinity method. Notably, Exo-spin™ buffer is much more efficient than generic PEG 6000 when used prior to Exo-spin™ midi column exosome isolation.
Data determined by nanoparticle tracking analysis (NTA). Each curve represents the average of 3 technical replicate measurements for each exosome isolation method triplicate experiment. (PM = Precipitation Method). Figure taken and adapted from (Martins, TS et al., 2018).
If you would like more information on the exosome extraction profile using Exo-spin™ midi columns, please refer to the EX04 technical note below in the documents section.
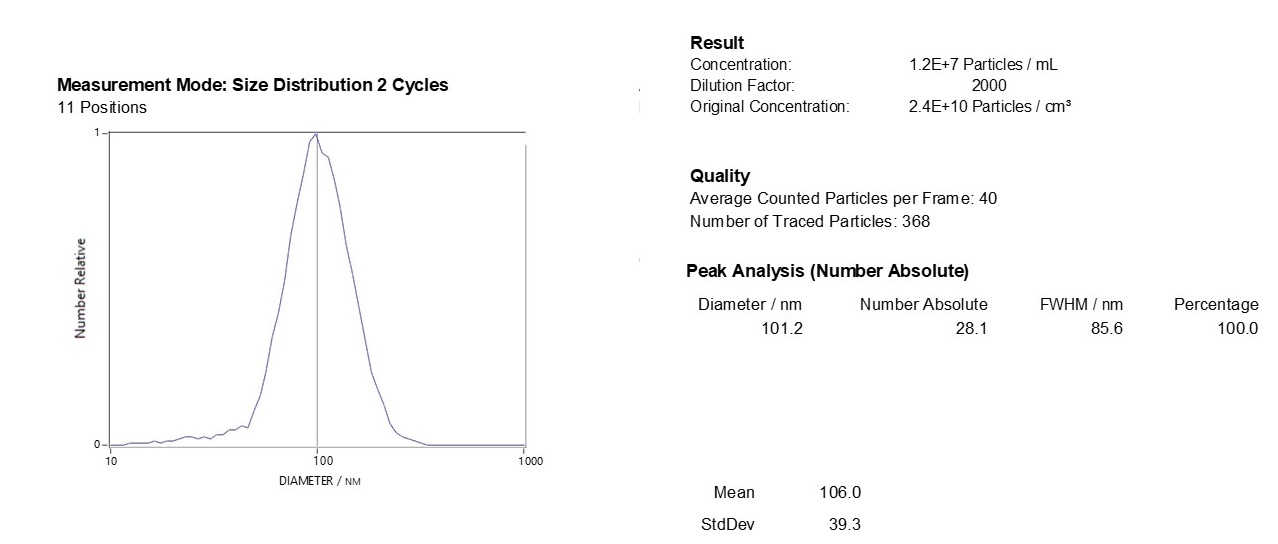
Characterizing Exo-spin™ isolated exosomes using nanoparticle tracking analysis (NTA)
Exosome characterization with NTA. Exosomes have been isolated using the Exo-spin™ kit and analysis performed with the ZetaView® instrument.
Downstream applications: advice and content
The EX04 Exo-spin™ kit is compatible with all downstream application and has been published in a large range of different applications.
RNA analysis:
- Santangelo L, Giurato G, Cicchini C, Montaldo C, Mancone C, Tarallo R, Battistelli C, Alonzi T, Weisz A and Tripodi M. (2016). The RNA-Binding Protein SYNCRIP Is a Component of the Hepatocyte Exosomal Machinery Controlling MicroRNA Sorting. Cell Reports 17(3):799-808.
- Brook AC, Jenkins RH, Clayton A, Kift-Morgan A, Baby AC, Shephard AP, Mariotti B, Cuff SM, Bazzoni F, Bowen T, Fraser DJ, and Eberl M. (2019). Neutrophil-derived miR-223 as local biomarker of bacterial peritonitis. Scientific Reports 9(1):10136.
Immunoassay:
- Salimu J, Webber J, Gurney M, Al-Taei S, Clayton A and Tabi Z. (2017). Dominant immunosuppression of dendritic cell function by prostate-cancer-derived exosomes. Journal of Extracellular Vesicles 6(1):1368823.
Protein assay:
- Welton JL, Webber JP, Botos LA, Jones M and Clayton A. (2015). Ready-made chromatography columns for extracellular vesicle isolation from plasma. Journal of Extracellular Vesicles 4:27269.
Application note:
Further resources:
Please see the Exosome Resources Page
Citations
There are more than 800 citations for Exo-spin products