Cancer Immunotherapy Models: The Right Scaffold Matters

Critical gaps between experimental models and clinical reality are the reason why clinical trials of promising drugs often fail. Closing those gaps can save huge amounts of money lost to failed trials. The right scaffold is critical to model accuracy
The CAR T-Cell Challenge in AML
CAR T-cell therapy has shown remarkable success against some blood cancers. However, its application to acute myeloid leukemia (AML) has been problematic. This is because targeting AML cells often means also attacking healthy blood cells that share the same surface markers, leading to dangerous side effects.
A promising workaround involves using CRISPR gene editing to remove these target markers from healthy cells before deploying CAR T-cells. This approach has worked well in simplified lab tests and animal studies, but there's been a persistent gap between these promising results and what actually happens in patients.

Schematic for the bone marrow niche model (taken from Doherty-Boyd et al)
The Need for a Better Model
The culprit behind this gap is surprisingly simple: the testing environment itself. Traditional cell culture involves growing cells on flat plastic surfaces in liquid media, a far cry from the complex, three-dimensional bone marrow environment where blood cells actually live.
PeptiGel: A Synthetic Bone Marrow Mimic
This is where PeptiGel becomes crucial. Developed from short self-assembling peptides, this synthetic (nature-mimetic) hydrogel can be tuned to match the mechanical stiffness of real bone marrow tissue. When researchers at the University of Glasgow tested different concentrations (ranging from soft to relatively stiff), they found that the firmest version, at 30 mg/mL, created an environment that mimicked the endosteal niche, the region where bone marrow meets bone.
Consistency Over Complexity
What makes PeptiGel particularly valuable is its consistency. Many existing bone marrow models rely on animal-derived materials like Matrigel or collagen, which vary widely from batch to batch and introduce biological complexity that muddles experimental results. PeptiGel, being fully synthetic and nature mimetic, eliminates this variability while still providing the physical cues cells need to behave naturally.
Creating the Right Cellular Environment
The Glasgow researchers discovered that mesenchymal stromal cells grown in PeptiGel systems expressed higher levels of nestin and stem cell factor, markers characteristic of cells in the native bone marrow niche. These cells then provided better support for maintaining hematopoietic stem cells in their undifferentiated state, a critical feature often lost in conventional culture systems.

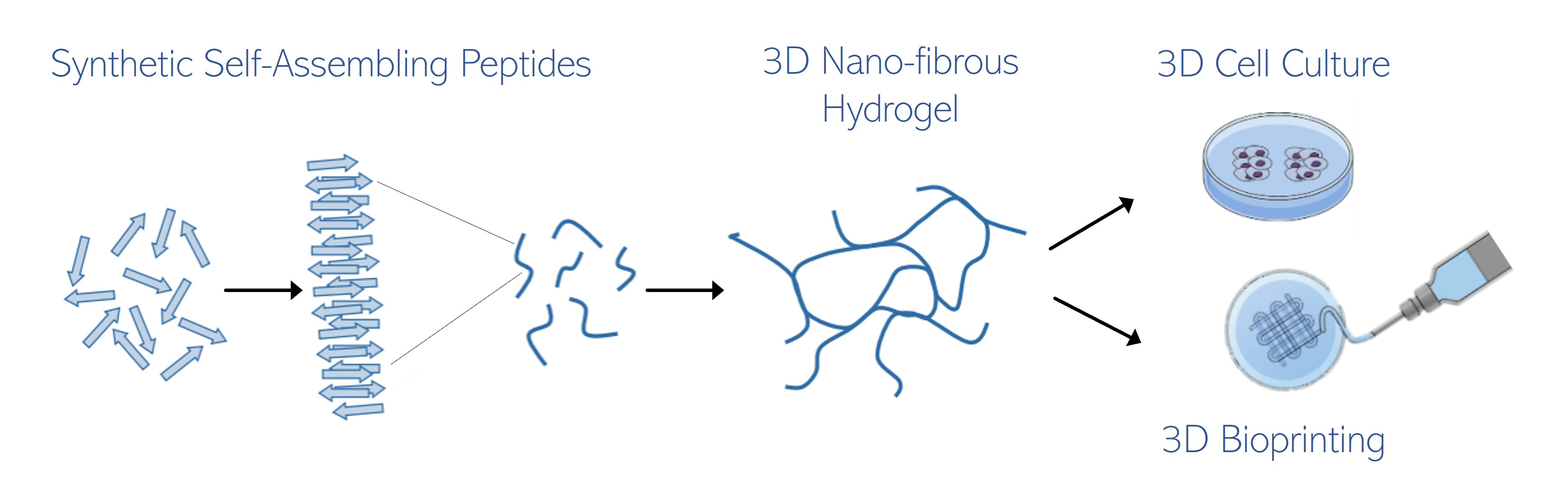
PeptiGel
Accurate Modelling
When the team tested their CRISPR-CAR T-cell therapy in both standard liquid culture and the PeptiGel-based model, the differences were striking. In liquid culture, the therapy appeared highly effective, completely eliminating AML cells. However, in the PeptiGel system, which more closely resembles actual bone marrow, residual cancer cells persisted when healthy stem cells hadn't been gene-edited first. This suggests conventional testing overestimates treatment effectiveness.
Perhaps more importantly, the PeptiGel model revealed off-target effects that liquid culture missed entirely. Even gene-edited stem cells showed some reduction in numbers when CAR T-cells and cancer cells were present together in the three-dimensional environment, suggesting unintended damage that wouldn't have been detected using standard methods.
Clinical Translation
This matters enormously for clinical translation. Therapies that look perfect in simple systems can fail or cause unexpected harm in patients. By bridging the gap between oversimplified lab tests and expensive, ethically complex animal studies, PeptiGel-based models offer a more predictive, reproducible platform for refining treatments before they reach human trials.
The synthetic nature of PeptiGel also opens doors for future applications. Researchers can systematically vary stiffness, incorporate specific bioactive molecules, or create high-throughput screening platforms, all while maintaining the reproducibility essential for therapeutic testing. For diseases originating in the bone marrow niche, having a reliable, human-relevant testing platform could accelerate the development of safer, more effective treatments.
IMAGE CAR-T therapy CREDIT CellGS
Learn more about powerful technologies that are enabling research:



