Can monocyte therapy end cancer's dominance over the immune system

In our immune system's fight against cancer, autologous immune cell therapy using CAR-T cells has transformed treatment options for patients with leukaemia and lymphoma but is not effective against solid tumors. Could monocytes and their derivatives, notably macrophages, succeed where CAR-T cells have failed? Here we look at the way cancers try to exert dominance over macrophages and the companies rising to the challenge of autologous macrophage therapy.
Macrophages
Macrophages are the multi-tools of the cell world. They rapidly transition between distinct states, changing their utility and activities depending on their environment and the challenges before them. In fact, a mosaic of macrophage phenotypes exists, with classic M1 infection-fighting and M2 tissue-rebuilding phenotypes, just two of the tiles in the mosaic. Macrophages can originate within tissues or can be derived from monocytes originating in the bone marrow.
Tumour Associated Macrophages
In cancer, monocytes are recruited in large numbers along chemokine gradients into the tumour. Once inside the cancer, they differentiate into macrophages or dendritic cells.
As key cells of the immune system, macrophages have developed to clear debris, fight infection, and attack challenges from foreign tissues, including cancers. However, cancer cells have an unstable genome which generates genetic variety within a cancer. This provides the raw material for immune editing whereby cells that are resistant to immune attack expand clonally. A growing cancer is one that has not only become resistant to immune cells but also gained control over the function of macrophages, subverting their behaviour to actively support tumour growth and spread. For example, a cancer cell that journeys from a primary tumour to establish distant metastatic outposts is accompanied on its journey by macrophages. To some extent, macrophages are immune cops led astray by the cancer.
Macrophages are often described as key orchestrators of the immune response directing the activity of other immune cells by the cytokines and chemokines they secrete.
Tumors can suppress monocyte activity through several mechanisms, which help them evade the immune system and promote tumor growth. Some of the ways tumors achieve this are:
- Secretion of Immunosuppressive Cytokines: Tumors often secrete cytokines such as IL-10, TGF-β, and VEGF, which can suppress the activation and function of monocytes and other immune cells.
- Induction of Myeloid-Derived Suppressor Cells (MDSCs): Tumors can promote the differentiation of monocytes into MDSCs, which are immunosuppressive cells that inhibit the activity of other immune cells, including T-cells and natural killer (NK) cells.
- Alteration of Monocyte Differentiation: Tumors can influence monocyte differentiation, leading to the development of tumor-associated macrophages (TAMs) that support tumor growth and suppress immune responses rather than attacking the tumor.
- Expression of Immune Checkpoint Molecules: Tumors can express molecules such as PD-L1 that interact with immune checkpoint receptors on monocytes, leading to their inactivation or exhaustion.
- Metabolic Reprogramming: Tumors can alter the metabolic environment, such as by depleting essential nutrients or producing metabolites like lactic acid, which can impair monocyte function and promote an immunosuppressive environment.
- Recruitment of Regulatory T Cells (Tregs): Tumors can attract Tregs, which are immunosuppressive cells that can inhibit monocyte activation and function through direct cell-cell interactions or by secreting inhibitory cytokines.
- Production of Extracellular Vesicles: Tumors can release extracellular vesicles containing proteins, lipids, and RNA that can modulate monocyte activity and promote an immunosuppressive phenotype.
- Expression of Anti-inflammatory Molecules: Tumors can express molecules that directly inhibit monocyte activation, such as CD47, which sends a “don’t eat me” signal to immune cells, including monocytes and macrophages.
- Hypoxic Environment: The low oxygen levels in the tumor microenvironment can affect monocyte function and promote the differentiation of monocytes into TAMs that support tumor growth.
- Induction of Apoptosis: Tumors can induce apoptosis (programmed cell death) in monocytes through the expression of Fas ligand (FasL) or other apoptotic signals, reducing the number of active monocytes available to mount an immune response.
- Modulation of Chemokine Expression: Tumors can alter the expression of chemokines, which are signalling proteins that guide the movement of immune cells. By modulating these signals, tumors can prevent the recruitment of monocytes to the tumor site or redirect them to other areas.
- Epigenetic Modifications: Tumors can induce epigenetic changes in monocytes, such as DNA methylation or histone modification, which can alter gene expression and suppress immune activity.
- Interference with Antigen Presentation: Tumors can interfere with the ability of monocytes to present antigens to T-cells, a critical step in initiating an adaptive immune response. This can be achieved by downregulating molecules involved in antigen processing and presentation.
Despite the control cancer exerts over the immune system, the key role of macrophages in cancer has led many researchers to explore how we can manipulate their function to create macrophage cell therapies to suppress or even defeat cancer. These therapies may be either autologous therapy using cells from the patient or syngeneic therapy, using cells from a donor. The advantage of autologous therapies is that the cells are perfectly matched to the recipient and won’t be rejected. The advantage of syngeneic therapies, particularly where manipulation of the cells is time-consuming or costly, is that the treatment is scalable, reducing cost and cells can be stored for immediate use. Companies are working to bridge the technological gap between autologous and syngeneic therapies by making the manufacture of autologous therapy as low cost and efficient as syngeneic therapy to generate cells perfectly matched to each patient.
The Challenges of Autologous Monocyte Therapy
The key challenges that need to be overcome when developing monocytes for therapy include
- Complex Biology: Monocytes are part of the innate immune system and can differentiate into various cell types, such as macrophages and dendritic cells. Understanding and controlling this differentiation process is complex.
- Heterogeneity: Monocytes are a heterogeneous population with different subsets that have distinct functions. Identifying and isolating the right subset for therapy is challenging.
- Short Lifespan: Monocytes have a relatively short lifespan in circulation, which can limit their therapeutic potential and the duration of their effects. The lifespan of their derivative macrophages in the tumour is about one-two weeks.
- Tumor Microenvironment: In cancer therapy, the tumor microenvironment can suppress monocyte (and derivative macrophage) activity or alter their function, making it difficult to achieve the desired therapeutic outcomes.
- Delivery and Targeting: Effectively delivering monocytes to the target site and ensuring they remain active and functional is a significant challenge.
- Immune Evasion: Tumors and other diseases may have mechanisms to evade or suppress monocyte activity, reducing the effectiveness of monocyte-based therapies
- Regulatory Challenges: Developing monocyte-based therapies involves navigating complex regulatory pathways to ensure safety and efficacy, which can be time-consuming and costly.
- Potential for Unwanted Immune Activation: Monocytes can potentially trigger unwanted immune responses, leading to inflammation or autoimmunity if not properly controlled.
- Limited Understanding of Mechanisms: There is still much to learn about the precise mechanisms by which monocytes exert their therapeutic effects, which can hinder the development of effective therapies.
- Patient Variability: Differences in patients’ immune systems, such as genetic factors or pre-existing conditions, can affect how modified monocyte therapies work, making it difficult to predict outcomes.
- Manufacturing and Quality Control: Ensuring consistent quality and functionality of monocyte-based products during manufacturing is challenging, especially when scaling up for clinical use.
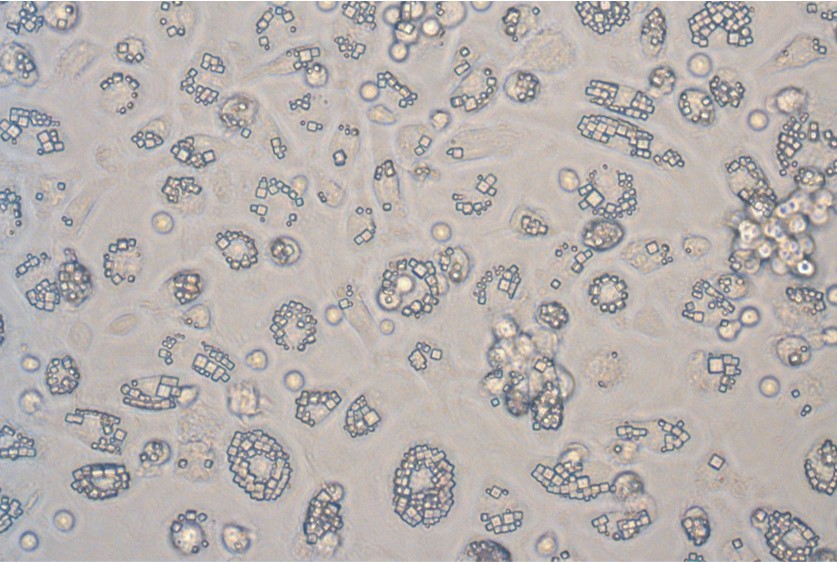
Monocytes engulfing sustained-release protein crystals to create cells for autologous therapy
These challenges are significant, but the potential clinical impact of monocyte cell therapies changing the lives of patients and their families is even bigger. Some of the companies developing monocyte and macrophage cell therapies include:
- Cell Guidance Systems: We are reprogramming monocytes by exploiting their efficient phagocytosis of sustained-release micron scale crystals. The tiny crystals are rapidly ingested by monocytes at a very high rate (>70%) within 24 hours. The ingested crystals secrete protein cargo that can change the behaviour of other cells. This approach provides a very simple way to move from cells provided by a patient to a therapy to start working on their cancer within a few days, and at a low cost. The therapy is being developed in collaboration with CRUK, initially to treat prostate cancer.
- Carisma Therapeutics: Specializes in engineered macrophage-based therapies, particularly for cancer treatment. Carisma uses CAN and RNA to reprogramme monocytes both in-vitro and in-vivo to create CAR-M cells. Collaborating with Moderna.
- Resolution therapeutics: manipulating macrophages to become pro-restorative primarily to target end stage liver disease.
Given the key role of macrophages in disease modulation and the success to CAR-T cells, it is perhaps surprising that there is so little monocyte therapeutics in development. This likely speaks to the challenges in the development of monocytes as cellular therapeutics rather than their therapeutic potential.
IMAGE CREDITS: (C) Cell Guidance Systems



