Genetic instability of iPSCs: Why does it matter?

The genomic instability of iPSCs is well documented. What is the impact of these genetic alterations on downstream applications and what can we do to mitigate their impact?
Through their unlimited capacity for proliferation and potential to differentiate into many cell types, induced pluripotent stem cells (iPSCs) have become a valuable tool for pharmacological screening and disease modelling. Moreover, the ethical advantage iPSC technology offers over embryonic stem cells (ESCs) and the feasibility of creating patient-matched pluripotent stem cells offer enormous potential for the development of regenerative therapies to treat a wide range of diseases. However, genomic instability of iPSCs is common and can significantly affect iPSC cell culture and potential downstream applications, particularly hindering the advancement of iPSC-based therapies.
There are at least three origins of genetic variation in iPSCs:
- The cloning of cells that occurs during iPSC generation may manifest pre-existing variations in parental somatic cells.
- Mutations induced by the reprogramming procedure.
- Mutations arising during prolonged culture of iPSCs, which are called passage-induced mutations.
Although a limited degree of genetic variation is expected, excessive genetic instability can be detrimental to the cells’ characteristics and functionality.
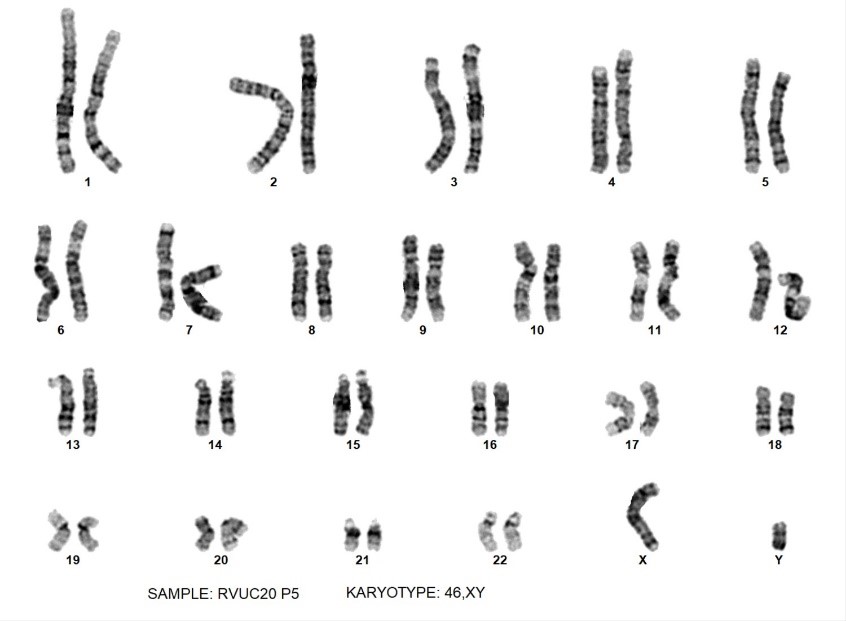
A typical normal human male karyotype from iPSCs - credit Cell Guidance Systems
One fundamental issue affecting IPSCs that, can be caused by genetic instability, is inconsistent or impaired differentiation. This is the result of altered expression of genes involved in maintaining pluripotency and differentiation. Alterations of such genes that provide a growth advantage is selected for in the culture. The effect of these genes' increased expression on differentiation pathways results in an inconsistent ability of iPSCs to differentiate into specific cell types, reducing the reliability of downstream assay data.
Accumulation of mutations can also increase the risk of malignant transformation upon transplantation into living organisms. Therefore, when considering the clinical application of iPSCs and developing iPSC-based therapies, genetic integrity is an overriding concern.
Another consequence of genetic instability is the development of a heterogenous population of cells within an iPSC culture. Different functional characteristics, growth rates and differentiation potential can all be consequences of the variability within a culture. This can cause complications with experimental design and interpretation.
A variation in proliferation rates and viability within an iPSC culture can be caused by genetic instability. This limits viable cell yields for experimental research but also poses a significant challenge for upscaling cell production for clinical applications. In addition, genetic alterations can compromise the functional characteristics of iPSCs. This could affect the cells’ ability to respond to environmental signals, compromising their use in disease modelling and drug testing. To mitigate these hazards and in order to reliably use iPSCs in research and therapeutic development, genetic integrity is paramount. Therefore, routine screening to assess genetic status is essential to monitor and maintain quality control.
Cell Guidance Systems offers karyotyping analysis, the gold standard of genetic audit required by regulatory agencies and scientific journals. Karyotype analysis is a cytogenetic test that can detect balanced translocations and copy number variations by utilising Giemsa staining techniques.
Our service for karyotype testing (sometimes called karyology) is most frequently applied to
- human karyotyping,
- mouse karyotyping (murine karyotyping)
We also have capabilities for
- Cow karyotyping (bovine karyotyping)
- Pig karyotyping (porcine karyotyping)
- Sheep karyotyping (ovine karyotyping)
- non-human primate karyotyping
- goat karyotyping (caprine karyotyping)
- Chicken karyotyping
- Dog karyotyping (Canine karyotyping)
- Horse karyotyping (equine karyotyping)
More information on the karyotype service is available here
Cell Guidance Systems also offers array analysis in the form of Array Genomic Hybridization (AGH). This provides a greater degree of resolution and can identify microdeletions that can be missed by karyotype analysis. Consequently, the combination of the two analyses allows you to achieve a comprehensive examination of genomic integrity. Together enabling rigorous control measures to help advance your iPSC-based research and therapeutic development.
More information on the AGH service is available here
MAIN IMAGE The origins of mutation in IPSCs CREDIT: Cell Guidance Systems Ltd



