Immune boot camps in cancer may explain the abscopal effect

A deadly arms race takes place between cancer cells and our immune system. A recent study published in Nature shows the importance of monocytes in the development of a healthy anti-cancer immune response. The study also demonstrates how cancers are able to suppress this response, and points to treatments that can further tip the balance in favour of the immune system.
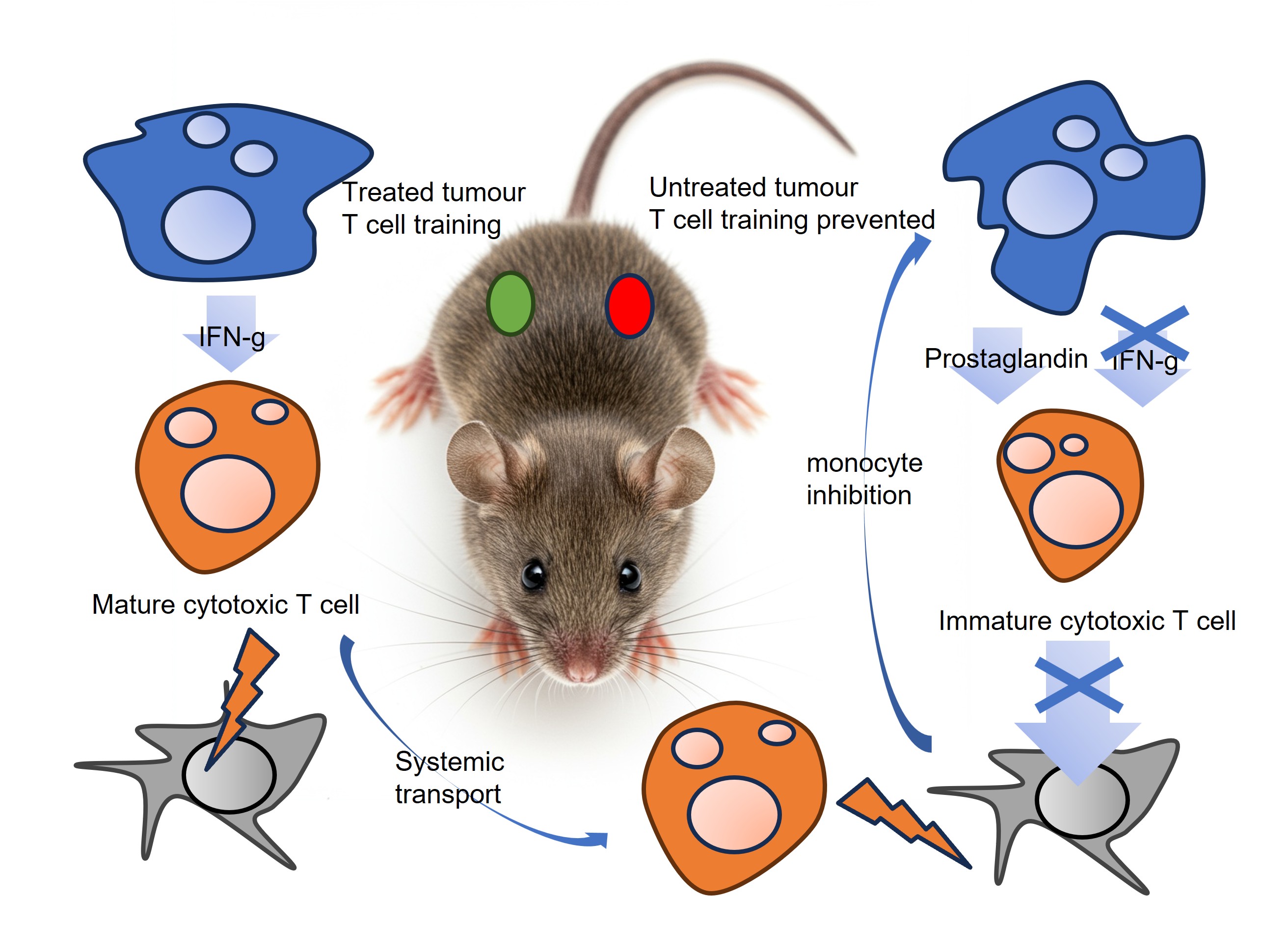
The immune system in cancer is organized into fighting units of different immune cell types. Members of the fighting unit include dendritic cells, T cells, monocytes and their derivative cells; macrophages and dendritic cells. As with soldiers in an army, immune cells receive basic training in isolation before banding together. Final immune cell training occurs in the cancer “battlefield” itself. Killer T cells are the key assassins who kill the cancer, but they are instructed by other immune cells including monocytes in boot camps located within the tumour. For example, monocytes instruct the T cells on how to recognize the cancer for targeted attacks.
Anna Obenauf and her colleagues at the Institute of Molecular Pathology in Vienna elucidated some of the ways in which cancer cells interfere with immune cell training. Their research provides important insights which may lead to rapid improvements in cancer therapy.
The researchers looked at the behaviour of immune cells in two related cancers. These were designed to model treatment-responsive and treatment-resistant cancer respectively.
(1) naïve tumours - untreated, still treatment-responsive
(2) resistant tumours – derived from the naïve tumours, but now unresponsive to treatment
Because these studies required a fully functioning immune system the bulk of the experiments had to be carried out in live mice. Mice could be given either or both types of cancer at the same time (in opposite flanks).
These studies identified two key mechanisms activated in the resistant tumours which block the training of T cells by monocytes:
- Prostoglandin secretion is increased by hyperactivation in cancer cells of MAPK signalling
- Type 1 interferons (e.g. IFN-gamma) production by monocytes was supressed
When mice were seeded with naïve cancer, in which these mechanisms were unaffected, they responded well to immunotherapy. When mice had treatment-resistant tumours, they responded poorly to immunotherapy (because of increased prostaglandin and reduced IFN). Interestingly, if a mouse had both naive and resistant tumours on distinct sites, the immune cells could undergo final training in the naïve tumour and effectively attack the resistant tumour located in the other part of the animal. This observation may explain the abscopal effect whereby treatment of a primary tumour can also shrink untreated metastatic sites: Immune cels can get training in the naïve tumour and then travel to fight in the resistant tumour. Consistent with the observations in the present paper. it has previously been suggested that boosting the immune system can boost the abscopal effect.
The abscopal effect may be achieved by immune cells being trained in treated or naive tumours and travelling to untreated tumours
Credit - Cell Guidance Systems
Cancer therapies work either directly by attacking cancer cells, or indirectly by boosting immune cells, or by preventing cancer’s suppression of the immune system. CAR-T cells and immune cell blockade inhibitors are widely used in cancer therapy. The insights gained in the latest research suggest that using COX inhibitors to reduce prostaglandin secretion could improve treatment efficacy. Fortunately, COX 2 inhibitors are already use in some cancer patients to manage pain. A retrospective analysis of clinical trials shows that patients who received COX inhibitors had improved treatment responses. Using COX-2 inhibitors more widely, and for longer, alongside immunotherapies, may provide a rapid way to improve clinical outcomes for checkpoint blockade inhibitors.
Addressing the blockade of interferon by cancer is also achievable. Similar to checkpoint blockade inhibition, antibodies could be used which block inhibition. This approach is akin to releasing the brakes in immune cell activity. But rather than releasing the brakes, it might also be possible to make the brakes redundant by engineering monocyte cells to constitutively express cytokines. This may eliminate cancers' ability to prevent T-cell training by monocytes.
At Cell Guidance Systems, we are at the forefront of efforts to reprogram monocytes using PODS, solid microscopic blocks of bioactive proteins, such as cytokines and chemokines, which are phagocytosed by monocytes and then sustainably secreted. We initially demonstrated the strategy using IL-2 to control melanoma tumour size.
IMAGE - Monocyte training of cytotoxic T cells. CREDIT: Cell Guidance Systems