Perfect EV purification?

Extracellular vesicles (EVs) are microscopic hollow lipid spheres which shuttle cargo, including proteins and nucleic acids, between cells. The biological importance of EVs in normal tissue homeostasis and also disease is well established. Consequently, their clinical potential as therapeutics and diagnostics is the focus of much attention.
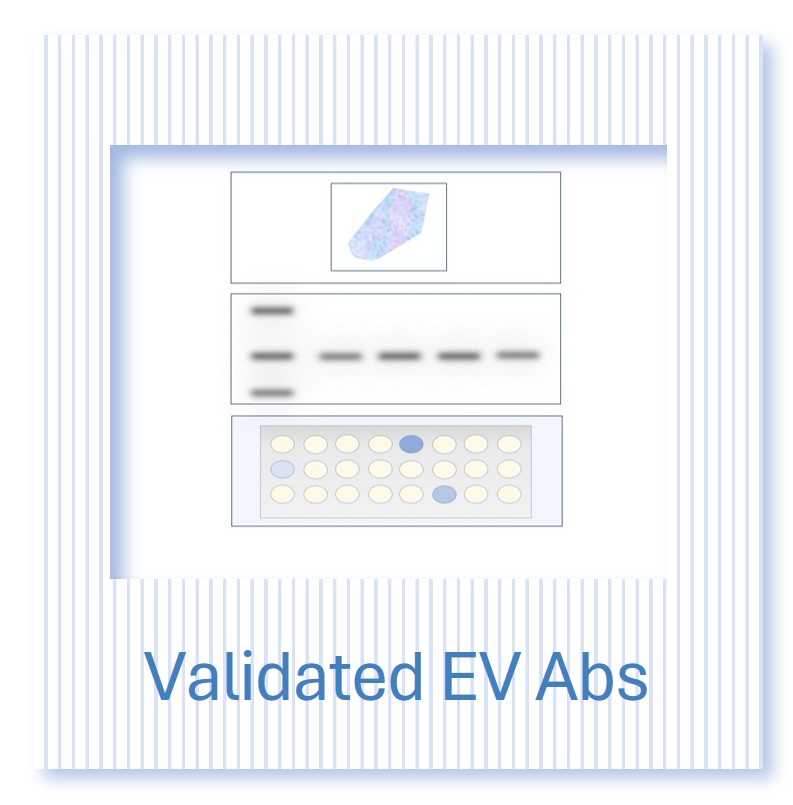
Several distinct EV categories have been identified. This complex nature presents particular challenges for characterization and classification. Working groups have published extensive guidelines on the minimal information set required to characterize EVs.
This process is important as it helps us better compare and standardize results between different labs and informs our understanding of EV function and biogenesis. Just as classifying cancer based on gene and protein expression profiles has led to tailored treatments, the classification of exosomes based on parameters such as size, marker proteins and nucleic acid content is defining distinct functional subsets. For example, based on size alone, EVs can be broadly divided into small exosomes (60-80 nm), large exosomes (90-120 nm), as well as the less-well-studied small EVs (150nm).
Purification of EVs is usually required prior to characterization and assay, and a wide range of EV purification techniques have been developed. These include ultracentrifugation, membrane affinity capture, size exclusion chromatography (SEC), precipitation, gradients, tangential flow and microfluidics. A combination of these techniques, notably precipitation followed by SEC, can also be used.
The purification method chosen is determined by a large range of factors which include:
- Yield
- Level of purity
- Access to equipment required (e.g. an ultracentrifuge)
- Cost
- Throughput
- Time required
- Technical difficulty
- Nature of EVs purified (which of the different EV classes based on size or other parameters)
- Specific downstream application requirements (e.g. avoid precipitants for mass spec analysis)
Ultracentrifugation was the workhorse in the early years of EV study. Indeed, a recent report from ISEV's Rigor and Standardization subcommittee which sruveryed exosome scientists identified ultracentrifugation as still the most widely used technique. However the report shows that since 2015 the use of SEC, such as Exo-spin from CellGS, has grown most rapidly and the number using SEC as grown from about 15% to almost 50% by 2019. THe reason is quite simple: For exosomes purification at least, SEC ticks the boxes in all the major selection criteria.
Whilst SEC looks set to surpass ultracentrifugation as the most widely used technique, there is no single technique that can satisfy all downstream requirements: a wide variety of alternative techniques enables different approaches to characterization and study of exosomes which may help drive the field forward.