Printing better celluarized blood vessels with PODS

In an exciting development, biomaterials scientists have used advanced biomaterials and an ingenious manufacturing method to produce co-axial extruded, cellularized blood vessels incorporating cells derived in situ from fat stem cells from the patient.
Damage to blood vessels from injury or disease can have serious medical consequences. When blood vessels cannot be repaired (e.g. with a stent), vascular transplant is a common procedure. Unfortunately, vascular surgery can lead to complications with a one year mortality rate of around 3%.
The most widely used procedure for surgical implants of veins is to transfer veins from one part of a patient to another. For example, in coronary artery bypass surgery, veins are typically sourced from one of the pateint's legs.
Autologous grafting has many drawbacks and limitations. For example, the availability of suitable autologous vessels can be limited, especially in patients with extensive vascular disease or previous surgeries that have already utilized potential graft sites. Harvesting autologous vessels can cause complications at the donor site, including pain, infection, and scarring. In some cases, the removal of a vessel can lead to functional impairment. The quality of the patient’s own vessels may be compromised due to age, disease (such as diabetes or atherosclerosis), or previous medical treatments. Poor-quality vessels can lead to graft failure.
Synthetic materials can be used instead of autografting. However, these materials tend to have higher rates of failure due to factors such as material-related thrombosis and they also tend to have higher rates of infection.
Regenerative medicine has the potential to provide better alternatives to autografts and synthetic veins by using patient-derived cells to engineer blood vessels. Blood vessels have a bilayer structure/ The inner surface is lined with endothelial cells. These are surrounded by a layer of smooth muscle cells. Engineering these bilayer tubular structures is technically challenging. Using innovative techniques and materials, researchers at University College London, led by Deepak Kalaskar, have demonstrated the feasibility of using a patient’s own fat as a starting material to produce cellularized vascular grafts.
Fat contains stem cells known as adipose-derived stem cells (ADSCs). These stem cells can be differentiated, with high levels of efficiency, to both endothelial cells and muscle cells when exposed to the growth factors VEGF165 and TGF-beta1 respectively. The researchers selected PODS® growth factors from Cell Guidance Systems. These provide sustained release and can be printed adjacent to particular cells to ensure optimal availability of these growth factors throughout the differentiation process.
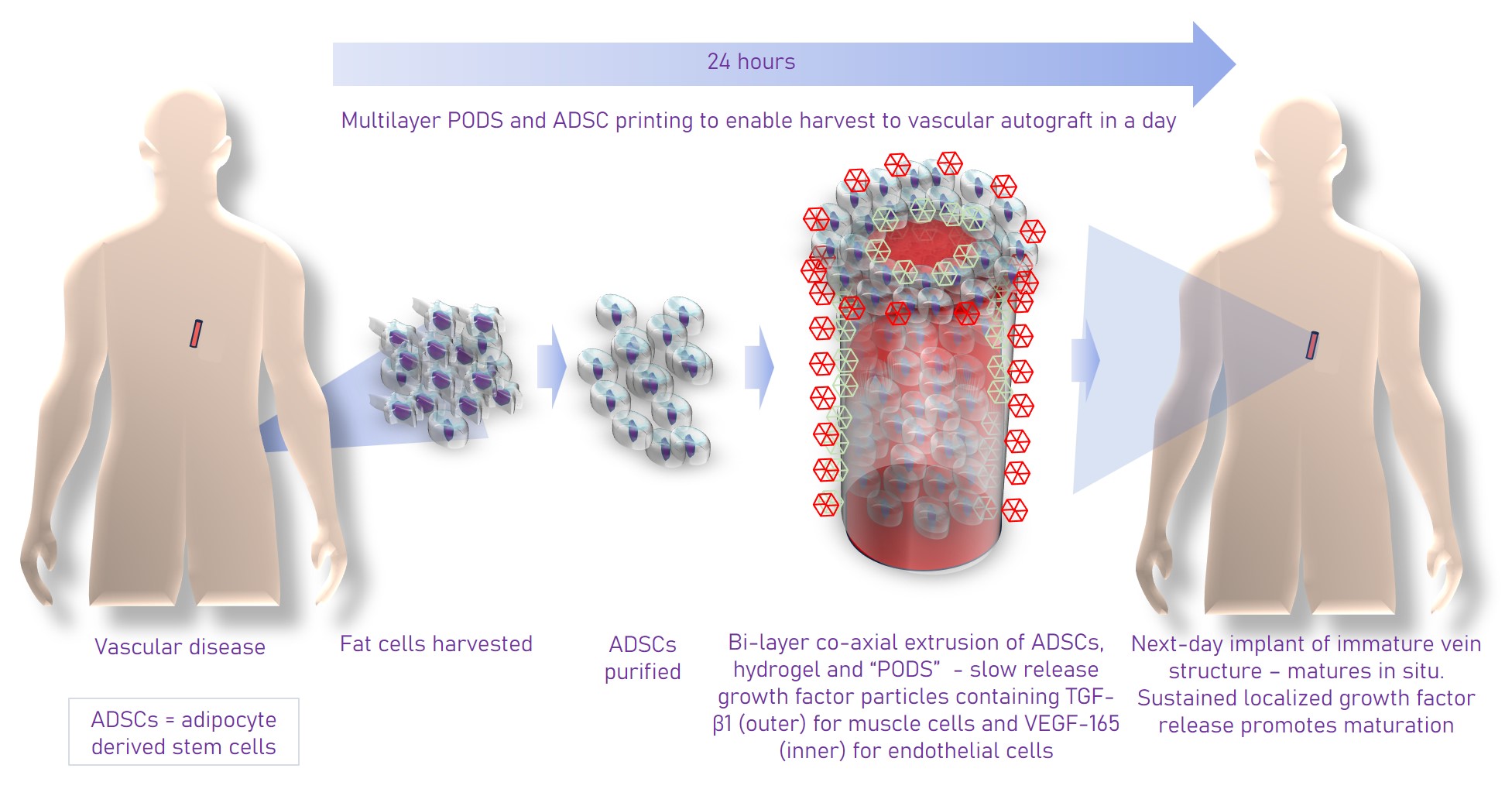
The researchers used a simple, yet elegant technique to fabricate the blood vessels. A coaxial printing process was employed using a proprietary hydrogel called VascuBiomatrix® which has excellent shear thinning properties and could be cross linked to form the outer layer of a tube. The inner layer of the tube was made of Pluronic-F127, a sacrificial material which was washed away once the outer layer was polymerized. The VascuBiomatrix® provided a scaffold for the cells. Rather than add these after printing, the ADSCs and PODS-TGFb1 were mixed with the VascuBiomatrix to differentiate in situ. The sustained release of growth factor from the PODS ensured high rates of differentiation to the muscle cells. Once the smooth muscle cells had formed, the muscle cell tube was irrigated with endothelial cells which had been formed in a cell culture dish by differentiating ADSCs with PODS-VEGF165. These cells colonized the inner surface of the tube formed of muscle cells. A wide range of analytical techniques, measuring gene and protein expression and assessing viability, was used to characterize the cells.
The printed blood vessels were also characterized with a CAM assay which uses chicken eggs to assess the ability of a substance or material to induce vasculogenesis. It was found that compared with blood vessels that only contain fat-derived stem cells, vessels containing the derived endothelial and smooth muscle cells induced much higher levels of vasculogenesis - hey to successful transplant. Another major advantage of the approach using sustained release PODS is that it opens up the possibility that the cells in the blood vessels can be differentiated and matured in situ, in the patient, accelerating the time between the harvesting of fat cells from the patient and implantation.
A webinar recording is available for this research.
PODS® used in bioprinting
IMAGE Scanning electron microscope images of co-axial extruded blood vessels CREDIT Priya et at



