Researchers see better results with formulated growth factors

Optimizing the formulation of drugs is critical to increase their stability and sustain bioavailability. Similarly, taking more care to formulate growth factors can transform their utility too.
Drug formulation
At high, saturating concentrations, drugs are toxic, and if the concentration is too low, the drug loses efficacy (see panel B above). The formulation of a drug is critical to maintaining a drug at optimal concentrations. The main tool for achieving this is excipients. These are inactive substances that serve as the vehicle and regulate the bioavailability of a drug. Examples of excipients commonly used for drugs include maize starch, magnesium stearate, and microcrystalline cellulose. Excipients provide:
- Stabilization: Excipients can help stabilize the active pharmaceutical ingredient (API) by protecting it from degradation due to environmental factors such as light, moisture, and temperature.
- Controlled Release: Excipients can be used to control the release rate of the active ingredient, ensuring it is delivered over a specific period.
Nanoparticle and microparticle technologies to regulate bioavailability and target drugs to specific tissues are widespread and have yielded significant improvements in drug performance, particularly for biological drugs.
Why growth factors need to be formulated
Whilst cell culture can indeed be made to work with unformulated growth factors (which are typically supplied as desiccated purified proteins, then resuspended in PBS or water), these unformulated growth factors have to be added in great excess to compensate for instability. Moreover, as these are periodically replenished during media changes, this creates gyrating growth factor concentrations in cell culture which stress cells.
The rationale for researchers tolerating the established practice of excess dosing of unformulated growth factors is based on the Gaussian response curve reported on a growth factor’s datasheet. These reported responses are generated with carefully chosen cell lines and assay readouts for which toxic effects on the growth factor at high concentrations are not apparent. However, growth factors are pleiotropic molecules which interact with different target cells and a cell’s particular pathways in ways that can’t be extrapolated from the assay shown in the datasheet. In fact, rather than producing a working range and working duration as shown in panel A in the main figure above, the effect of growth factors may be more closely represented by panel B.
PODS® microcrystalline formulation
PODS® are formulated growth factors that use in-cell microcrystalline protein scaffolds to stabilize and slowly stream growth factors over 4-8 weeks.
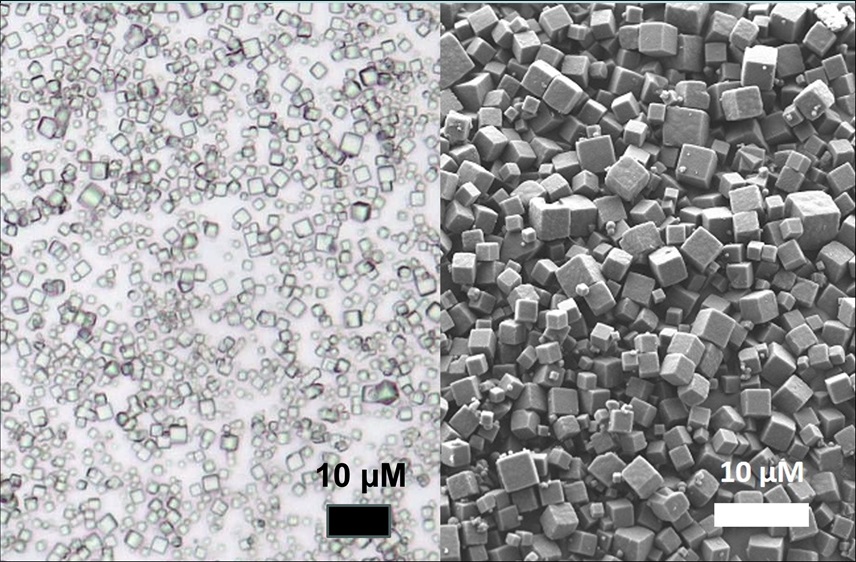
PODS® formulated growth factors seen under light and scanning electron microscopy. Image credit. Cell Guidance Systems
The effect of using PODS®
Neuronal cells are notoriously sensitive, and the effect of increasing stability is well illustrated in neuronal culture. For example, the maturation of otic neuronal cells from the inner ear shown below is clearly superior when cultured with PODS® BDNF compared to unformulated BDNF. In this case, both neurite length and degree of arborization (branching) are significantly increased with PODS®.
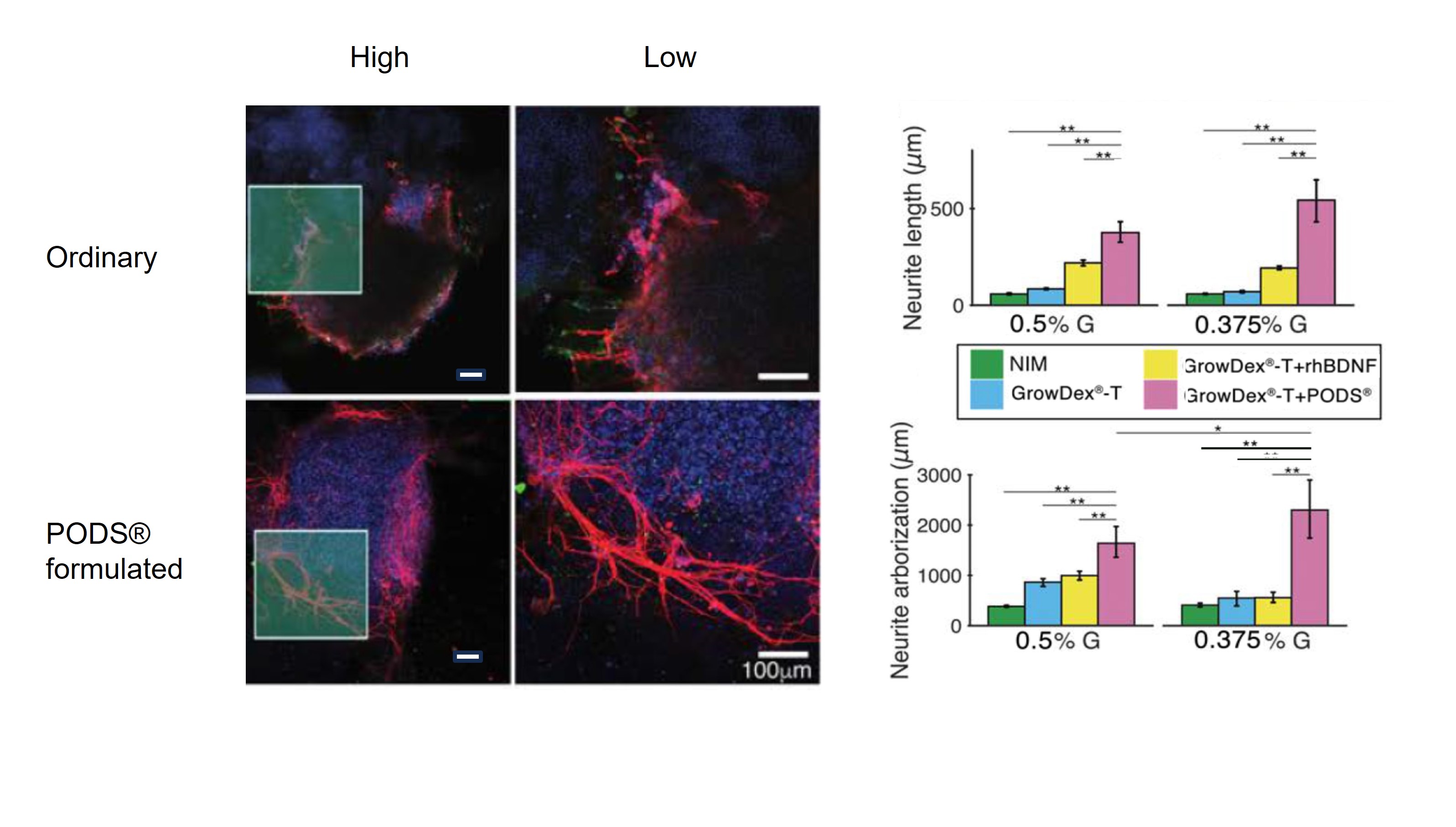
Otic neuronal cell spheroids shown under high and low magnification treated with ordinary BDNF (top) or PODS® BDNF (bottom). Neurites stained in red are clearly longer and more branched with PODS®. This is quantified in the panel on the right. Image credit Akihiro Matsuoka, Northwestern University
If you would like to experience the benefits of PODS® in your cell culture, please get in touch.
MAIN IMAGE Effective concentration ranges of bioactive molecules without toxicity (A) and with toxicity (B) CREDIT: Cell Guidance Systems Ltd
Learn more about powerful technologies that are enabling research:



