Vestibular neuron breakthrough enabled by PeptiGel and PODS

In a technology breakthrough utilizing both PODS® and PeptiGel®, researchers at the University of California in San Diego have used a microfluidic approach to accurately generate vestibular nerve cells to improve electrical vestibular stimulation (EVS) prosthetic outcomes.
The vestibular system, together with the cochlea, forms the inner ear in humans and most other mammals. The vestibular system provides us with our sense of balance and orientation. Vestibular neurons (VNs) carry the information from the vestibular system to the brain.
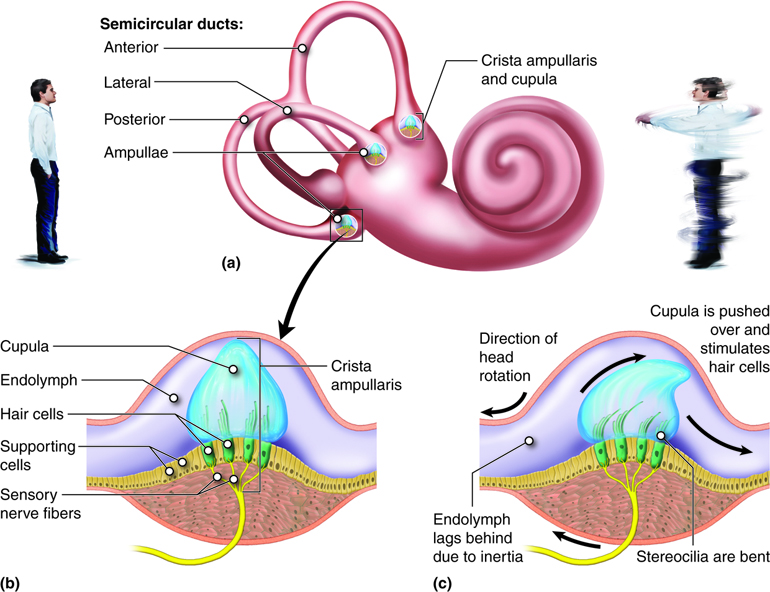
Structure and Function of the Vestibular system. Three canals each have an ampulla containing a crista ampullaris and cupula (a). When the head is stationary, the cupula, and embedded stereocilia, are not bent (b). When the head rotates in the same plane as one of the canals, the fluid in the canal (endolymph) lags, leading to bending of the stereocilia in the cupula, which initiates nerve impulses. Image by Cenveo is licensed under a Creative Commons Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/).
Millions of people live with damage to the vestibular system of both ears resulting in a range of symptoms including loss of balance. Recently, EVS prosthetics have offered innovative solutions but these prosthetics tend to form poor connections with the brain severely impacting their performance.
A connecting bridge of new neurons to integrate the EVS can be expected to improve EVS performance. However, growing VNs has been challenging. Now, a system to grow vestibular neurons in the lab, published in Tissue Engineering, has been developed by Akihiro Matsuoka and colleagues at the University of California in San Diego (UCSD).
In the developing embryo, VNs form alongside the neural tube (which gives rise to the early brain and spine). The proximal and distal sides of this tube secrete the growth factors Wnt3a and SHH which affect the development of the VNs. To recapitulate this environment in vivo as they generate VNs from iPSCs, the UCSD group built on the foundational work of others developing a complex, stepwise programme of differentiation which generates otic vestibular progenitor spheroids as a precursor to the VNs. In addition, the researchers utilized a microchannel device which allowed the generation of a Wnt3a-SHH concentration gradient.
The group had previously identified Growdex and PeptiGel® Gamma 2 as the only hydrogels that worked in a system to generate otic neuronal cells and used them both in the present paper to equal effect.
The authors point out that the ability to generate extended neurites will be critical to any therapeutic application of this technology with a gap of 3-7 mm needing to be bridged. As with previous studies, the use of PODS®-BDNF, a depot formulation which provides sustained BDNF release over several weeks, significantly outperformed conventional BDNF leading to markedly enhanced axon and neurite formation.
How PODS® and PeptiGel® can help your research
PODS® are increasingly used in leading labs to address the limitations of conventional growth factors. Unlike other sustained release systems, they do not suffer from burst release. They have proven themselves as therapeutics in numerous in-vivo models of disease.
PeptiGel® is a family of related self-assembling peptide hydrogels. PeptiGels® provide a tailored approach to matrix for 3D culture of cells and organoids.
MAIN IMAGE: From Norton et al, 2023
Learn more about powerful technologies that are enabling research:



