Virus-like particles better than AAV?

A recent paper exploring the use of optimized virus-like particles (VLPs) to deliver base editing proteins has shown impressive levels of efficacy and, importantly, low levels of off-target activity in mouse models of therapy for the brain, eye, and liver disease.
The delivery and release of therapeutic drugs to their target cells is a critical aspect of drug development. Each drug has a specific therapeutic window: to be effective, a therapeutic drug needs to be delivered to target cells at an effective concentration for a specified duration. If the drug is a protein, and the target is intracellular, this development task can be particularly challenging.
Virus-like proteins (VLPs) are structures that contain multiple copies of virus proteins that self-assemble into a virus-like structure. Externally, VLPs resemble viruses, and VLPs can employ the same mechanisms as viruses to enter target cells. However, unlike virus, VLPs lack viral genetic material, and are therefore unable to replicate in the target cell.
VLPs can be engineered to contain cargo molecules including nucleic acids, proteins and small molecules and have attracted widespread interest for their potential use in drug delivery. Similar to viruses, VLPs can be modified to modulate their tropism (their preference for entering particular target cells) to enable targeting of specific cell types.
Recently, a team of researchers led by David Liu at Harvard University reported on their efforts to develop engineered VLPs as protein drug delivery vehicles. The researchers were specifically interested in the potential of VLPs to carry base editors (BEs). These are proteins such as Cas9 which can identify and correct DNA point mutations and rectify disease-causing genes.
Adeno-associated viruses (AAVs) have been the method of choice for delivering BEs to target cells in-vivo. However, AAVs have two key drawbacks compared to VLPs: Because they replicate, they can persist and generate prolonged protein expression leading to increased risk of off-target effects (editing non-target genes). In addition, AAVs have the potential to integrate into host DNA which can be oncogenic. In contrast, VLPs can deliver the therapeutic protein (or ribonucleoprotein) for a shorter period of time, reducing the frequency of off-target base edits. Moreover, VLPs are inherently safer as they don’t carry nucleic acids that can integrate into the host genome to cause cancer.
VLPs carry their cargo protein internally as a fusion protein with the gag envelope protein (which forms part of the capsid shell of viruses and VLPs). A linker sequence between the cargo and gag proteins includes a sequence that enables cleavage of the cargo protein after reaching the target cell cytosol.
A principal challenge with VLPs has been to engineer them to contain sufficient cargo that is optimally cleaved for deployment, bearing in mind that cleavage that is too efficient may hinder initial incorporation into VLPs. Another consideration for BE proteins is the presence of nuclear localization signals. These enable BE to reach the nucleus to edit target DNA. However, during VLP manufacture in producer cells, these would prevent BE proteins from accumulating in the cytosol which is essential for incorporation into VLPs. The researchers optimized the cleavage signals and, to promote localization in the cytosol, added nuclear export signals. These were titrated for optimal effect and positioned so they would eventually be cleaved off in the target cell but leave the BE nuclear localization signals intact to direct translocation to the cell nucleus.
The final tweak the team made was to optimize protein stoichiometry. The researchers did this by titrating the amounts of wild-type gag-pro-pol proteins against the gag-cargo protein by modulating the amount of plasmid encoding each protein used for transfection of the VLP production cells. They settled on a ratio that provided a good trade-off between the number of cargo proteins per particle and overall VLP titers.
The final version of the VLP achieved 5.6-fold higher levels of base-editing with very low levels of off-target editing in cell lines grown in-vitro. Importantly, when VLPs were used to deliver VLPs in vivo to the brain targeting the DNMT gene (via intrathecal-like injection), to the liver to target the PCSK9 gene (via systemic intravenous injection), and to the eye targeting the Rpe65 gene (via sub-retinal injection), impressive rates of base editing, up to 24-fold higher than existing VLPS and comparable to those obtained with AAV delivery of the base editing proteins, were seen in each case. But unlike AAV, off-target bystander conversion rates were minimal. With IV injection for the liver, the tropism of the VLP enabled specific delivery to the liver with low levels of base correction in the spleen and background levels in other tissues.
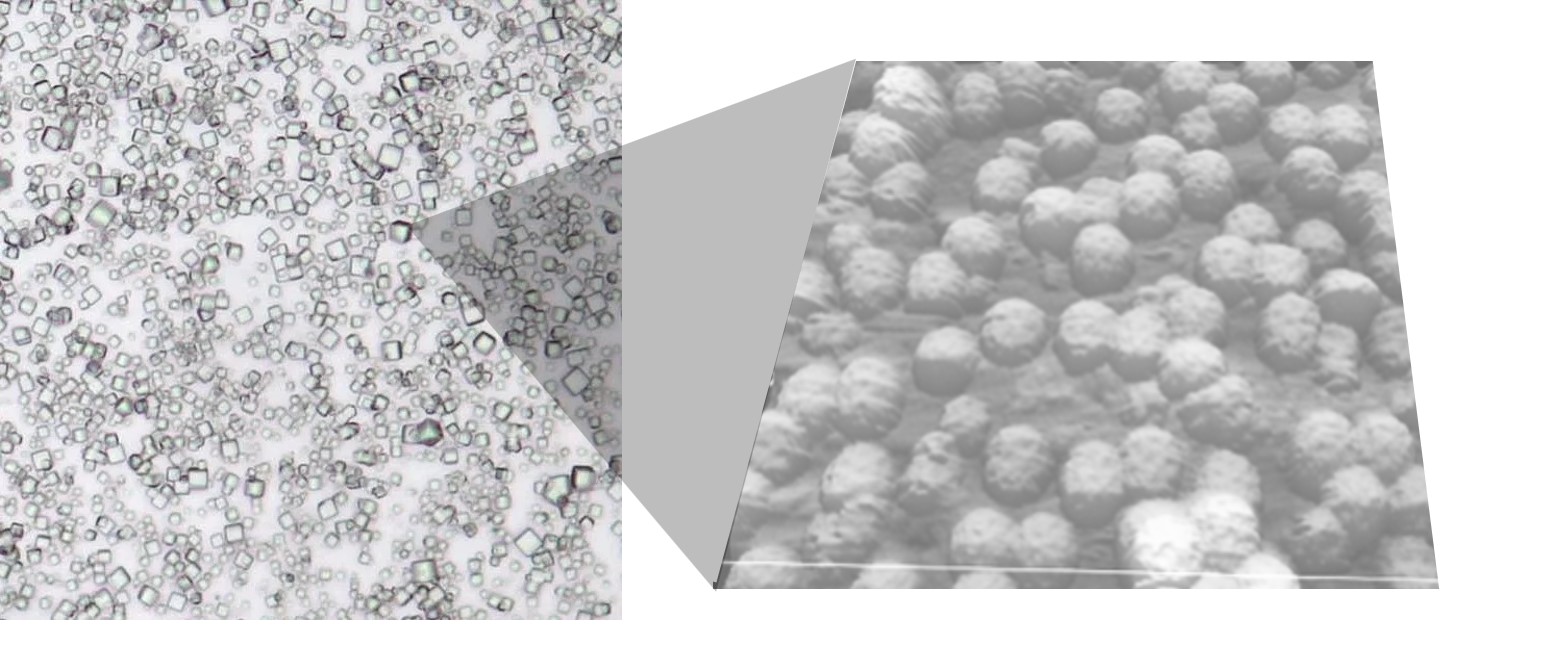
PODS crystals have been engineered to contain VLPs.
This work is a tour de force. The authors’ systematic process of refinement was able to transform the efficacy levels for VLPs for delivering BE proteins. But what about the many cargos which require more prolonged exposure to therapeutic proteins? Although able to provide durable delivery, doubts remain about AAV’s oncogenicity. VLPs could be administered repeatedly, but this is not always practical. One possible solution is to use a delivery system that provides localized, sustained release of VLPs. PODS crystals originate from a mechanism to package and protect cypovirus particles so are readily able to package VLPs for sustained release. As shown in the image above, norovirus VLPs can be packaged in PODS. Furthermore, since PODS are readily taken up by monocytes which home in on sites of disease, PODS-VLPs could provide a powerful mechanism for targeted, sustained, cytosolic delivery of drugs either following direct injection or via a cell-mediated Trojan horse drug delivery approach enabling delivery across the blood-brain barrier.
IMAGE: Norovirus VLPs encased in a PODS crystal viewed with scanning tunneling microscopy. Credit: Hajime Mori
