The enemy within: immune cell invading pathogens

As pathogens evolve, they develop “ingenious” survival strategies. Perhaps some of the most fascinating are the pathogens that have hit upon the strategy of taking up residence in the very cells tasked with their termination: phagocytic immune cells. This strategy also makes the task of clearing infections using drugs very challenging.
The mononuclear phagocytic cells of the immune system are capable of engulfing foreign cells and cellular debris to prevent and reduce infections. These include dendritic cells and monocytes which can differentiate into the larger macrophages. These cells primarily originate as precursors in bone marrow, and in their mature form, they are found throughout the body and play critical roles in fighting invading pathogens.
Cell-penetrating protozoans
A variety of unicellular organisms have utilised the phagocytotic ability of these cells against their host organisms. These parasites allow themselves to be deliberately engulfed or will actively invade immune cells. Furthermore, some of these parasites are capable of modifying the invaded cell’s signalling pathways, changing the migratory behaviour to use them as a form of transport for their wider dispersal.
Unicellular parasites can be divided into those which must reside within cells to complete their lifecycle (obligate intracellular parasites) and those capable of living and reproducing either inside or outside cells (facultative parasites). Plasmodium, Trypanosoma, Leishmania and Toxoplasmosis belong to the obligate intracellular parasites and are some of the most well-studied protozoa with global health impacts to humans.
Disease Impact of obligate parasites
Plasmodium, the most well-known of these parasites, is the underlying cause of malaria. If not promptly treated it can cause kidney failure, seizures and ultimately result in coma, and death. The World malaria report, estimates there were 247 million malaria cases in 2021 causing over 600,000 deaths.
Leishmaniasis, caused by the eponymous Leishmania parasite, has two common forms: cutaneous (causing skin sores) and visceral (affecting several internal organs- usually spleen, liver, and bone marrow). According to the WHO, Leishmania results in 700,000 to 1 million new cases annually.
Trypanosomiasis makes up several diseases caused by parasitic protozoans of the genus Trypanosoma. One of the most well characterised in terms of its life-cycle is T. cruzi which causes Chagas disease. It is estimated that 6 to 7 million people worldwide are infected. In the chronic phase of infection, T. cruzi can cause gastrointestinal complications and heart problems such as irregular heartbeat and failure resulting in sudden cardiac arrest.
The above parasites are all transmitted to humans and other mammals through blood sucking insects where the first stage of the parasite’s life cycle is completed.
On the other hand, Toxoplasmosis (caused by T. gondii) is usually acquired through consumption of contaminated food (although cats are the primary hosts of this parasite, and so transmission can occur via close contact with its faeces). The most severe effects are seen in pregnant women. If infection occurs during or just before pregnancy, it can result in miscarriage, stillbirth or child disability. It is estimated that every year there are over 1 million cases of toxoplasmosis in Europe caused by contaminated food. According to the Centre for Disease control, in the USA more than 40 million people (>10% of the population) carry the parasite. Most of these individuals are asymptomatic.
Mechanisms of invasion
Plasmodium sporozoites are equipped with specialized mechanical proteins which allow them to rapidly traverse skin cells and find their way to the bloodstream after being injected into the body by the female mosquito. Once in the blood, these sporozoites are transported to the liver where they ultimately invade hepatocytes. However, on their way to reaching the hepatocytes they must first traverse the liver endothelium which is lined with Kupfer cells (liver-resident macrophages). The Kupffer cells actively engulfing the parasite via invagination of the cell membrane. The sporozoites are able to modulate Kupffer cell function forcing them to undergo programmed cell death thus immunosuppressing the cellular microenvironment.
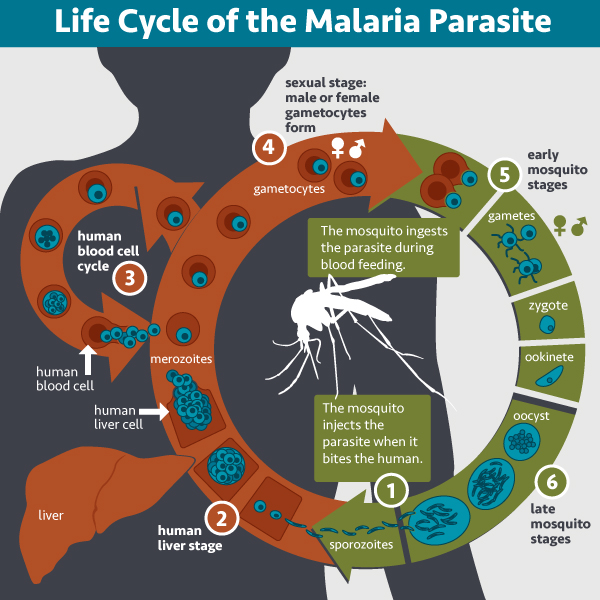
Life Cycle of the Malaria Parasite CREDIT: NAID
Ultimately, the sporozoites arrive at the hepatocyte, actively invading these cells and forming a parasitophorous vacuole, shielding it from the immune system to avoid degradation by the cell’s endocytic/lysosome systems. Here it completes the next stage of its development into the merozoite which prepares it for the blood stage of the lifecycle. When this stage of development is accomplished, Plasmodium exits the liver cells disguising itself in the host cell’s membrane to form a structure known as a merosome. The merosome provides as a cloak of invisibility to avoid detection by macrophages and enabling the parasite to target and take up residence in the circulating red blood cells. Once inside the red blood cells (RBCs), the merozoite undergoes transformation into the ring-shaped trophozoite. It then undergoes several rounds of asexual reproduction, each time the clones erupt from the RBCs to invade new ones. This blood stage is where the characteristic clinical symptoms of malaria are seen and those parasites, which successfully evade destruction by the host’s immune system, are then eventually taken up the mosquito when it feeds, this completing the lifecycle.
Other parasites are taken up by circulating mononuclear phagocytes at the site of injection. The Leishmania promastigotes are initially engulfed by neutrophils which are then phagocytosed by macrophages and dendritic cells. With Trypanosomes, the metacyclic trypomastigote stage is engulfed by macrophages and dendritic cells. In both of these cases, the immune cells provide concealment for the completion of the next stage of development. In Leishmania, engulfment is initiated by the promastigote binding to the surface of the neutrophil. Upon entering the host cell, a parasitophorous vacuole also envelops the Leishmania preventing acidification and the triggering of the microbicidal machinery. This enables sufficient time for promastigotes to differentiate into amastigotes.
As well as exploiting the actin-dependent uptake mechanism in phagocytic cells, Trypanosomes will use cell surface attachment signalling pathways to trigger entry into non-phagocytic cells. Both routes of entry are used in macrophages and dendritic cells, though it is unclear whether one method predominates. In addition to providing refuge from the body’s immune response, there is the additional benefit that, after engulfment, these professional phagocytes will migrate away from the site of infection thereby facilitating dispersal of the parasite.
This function of dispersal has been exploited further by T. gondii. After being ingested, the disseminating stage (known as the tachyzoite) traverses the intestinal epithelium to reach the blood stream and enter circulating leukocytes particularly dendritic cells and macrophages. The dendritic cells distribute the tachyzoites, acting as a “Trojan horse”.
In an interesting further twist, Toxoplasma parasites induce expression of dendritic cell-associated transcription factors in macrophages. This appears to reprogramme the macrophages to become more like dendritic cells and respond to chemotactic signals actively migrating throughout the body enabling dispersal.
Therapeutic opportunities
These different mechanisms of infection make finding a single therapeutic approach very challenging. In addition, drug resistance of the parasites to existing drugs is one if the major issues for current therapeutics, particularly for malaria. This, alongside the toxicity often seen with some of the anti-leishmanial drugs fuels the need to discover and approve new interventions and approaches. The different modes of invasion in different cells will most likely need multicomponent drugs against the various molecular components of invasion. Furthermore, another avenue could be to target the immune cells themselves to take up drugs via nanocarriers. Utilising the phagocytic mechanism to deliver drugs intracellularly has the potential to selectively target the parasite itself circumventing side effects.
Main IMAGE Colorized electron micrograph showing malaria parasite (right, blue) attaching to a human red blood cell. The inset shows a detail of the attachment point at higher magnification.
Credit: NAID



